Study Finds That Both Bacterial Cytolethal Distending Toxin And SARS-CoV-2 Uses Cellugyrin (Synaptogyrin-2) Dependent Pathways To Gain Cell Entry!
Nikhil Prasad Fact checked by:Thailand Medical News Team Apr 22, 2024 1 year, 4 days, 1 hour, 42 minutes ago
COVID-19 News: The intricate interplay between pathogens and host cells has long been a subject of intense research, driven by the need to understand how bacteria and viruses breach cellular defenses to cause infection and disease. Recent advancements have shed light on the role of cellugyrin (synaptogyrin-2), a critical component in cellular trafficking, in facilitating the entry of both bacterial toxins and viruses into host cells. This
COVID-19 News report delves into a groundbreaking study conducted at the University of Pennsylvania, Philadelphia, USA, exploring the shared pathways exploited by these pathogens and the implications for therapeutic interventions.
 Both Bacterial Cytolethal Distending Toxin And SARS-CoV-2 Uses Cellugyrin (Synaptogyrin-2) Dependent Pathways To Gain Cell Entry!
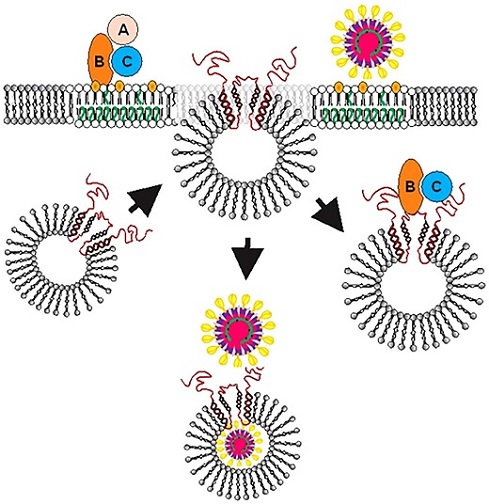
Proposed model for the role of SLMVCg+ in facilitating Cdt or viral entry into host cells and intracellular trafficking. After binding of toxin (Top left) or virus (Top right) to lipid raft associated receptors, SLMVCg+ translocate from cytoplasm to plasma membrane allowing cellugyrin (red) to be exposed to the extracellular surface and associate with pathogen binding proteins. For viruses such as SARS-CoV-2 we further propose that a second step (also occurring in close proximity to the plasma membrane) involves fusion events that ultimately lead to viral translocation to the SLMVCg+ lumen. Orange represents cholesterol and green unsaturated fatty acids within the host cell membrane.
Understanding Cellugyrin and Synaptic-Like Microvesicles (SLMVs
Both Bacterial Cytolethal Distending Toxin And SARS-CoV-2 Uses Cellugyrin (Synaptogyrin-2) Dependent Pathways To Gain Cell Entry!
Proposed model for the role of SLMVCg+ in facilitating Cdt or viral entry into host cells and intracellular trafficking. After binding of toxin (Top left) or virus (Top right) to lipid raft associated receptors, SLMVCg+ translocate from cytoplasm to plasma membrane allowing cellugyrin (red) to be exposed to the extracellular surface and associate with pathogen binding proteins. For viruses such as SARS-CoV-2 we further propose that a second step (also occurring in close proximity to the plasma membrane) involves fusion events that ultimately lead to viral translocation to the SLMVCg+ lumen. Orange represents cholesterol and green unsaturated fatty acids within the host cell membrane.
Understanding Cellugyrin and Synaptic-Like Microvesicles (SLMVs)
Before delving into the specifics of pathogen entry, it's essential to grasp the role of cellugyrin and SLMVs in cellular processes. Cellugyrin, a member of the synaptogyrin tetraspanin protein family, plays a crucial role in vesicle biogenesis, exocytosis, endocytotic recycling, and neurotransmission. It is widely expressed across various tissues, making it a ubiquitous player in cellular trafficking. SLMVs, specifically SLMVCg+, represent early sorting vesicles containing proteins essential for endocytic processing and likely contribute to the trans-Golgi network (TGN) and lysosomal transport. The dynamic interplay between cellugyrin and SLMVCg+ forms the foundation for understanding pathogen-cell interactions.
Unraveling Bacterial Toxin Entry: The Case of Cytolethal Distending Toxin (Cdt)
Aggregatibacter actinomycetemcomitans cytolethal distending toxin (Cdt) serves as a model for bacterial toxin entry mechanisms. Cdt intoxicates a range of cell types by binding to cholesterol-rich membrane lipid rafts, triggering the internalization and trafficking of its subunits (CdtB and CdtC). Notably, cellugyrin's presence is indispensable for Cdt-induced toxicity, as demonstrated by cell lines with impaired cellugyrin expression showing resistance to Cdt-mediated effects. This dependency on cellugyrin highlights its crucial role in facilitating bacterial toxin entry and subsequent cellular damage.
;
Parallels with Viral Cell Entry: Insights from SARS-CoV-2
The emergence of SARS-CoV-2, the virus responsible for the COVID-19 pandemic, brought a renewed focus on viral cell entry mechanisms. Like bacterial toxins, SARS-CoV-2 exploits cholesterol-rich lipid rafts and cell surface receptors (e.g., ACE-2) for cellular entry. This shared modus operandi prompted researchers to investigate whether cellugyrin plays a similar role in viral cell entry pathways.
Experimental Approaches and Key Findings
Experimental approaches included the generation of cellugyrin-deficient cell lines and the assessment of their susceptibility to viral infection using pseudotype viruses mimicking SARS-CoV-2. Remarkably, cells lacking cellugyrin expression exhibited resistance to viral infection, underscoring the pivotal role of cellugyrin in mediating SARS-CoV-2 entry.
The culmination of these all the studies yielded several key findings that significantly advanced our understanding of cellugyrin-dependent pathways in pathogen entry:
-Cellugyrin Dependency: The study unequivocally demonstrated the dependency of both bacterial toxins, exemplified by Cdt, and viral pathogens like SARS-CoV-2 on cellugyrin for efficient cellular entry. Cell lines lacking or with reduced cellugyrin expression exhibited marked resistance to pathogen-induced toxicity or infection, highlighting the indispensability of cellugyrin in these processes.
-Mechanistic Insights: Through binding assays and domain mapping studies, researchers uncovered specific regions within cellugyrin peptides that exhibited preferential binding to pathogen components. This provided crucial mechanistic insights into the molecular basis of pathogen-cellugyrin interactions, offering potential targets for therapeutic interventions aimed at disrupting these interactions.
-Pathway Crosstalk: The study also revealed intriguing crosstalk between cellugyrin-dependent pathways and cellular signaling cascades. Alterations in cellugyrin expression not only influenced pathogen entry but also impacted downstream signaling events, including immune responses and cellular stress pathways. This cross-functional role of cellugyrin underscores its significance beyond vesicle trafficking, extending into pathogen-host interactions and cellular homeostasis.
Molecular Interactions: Mapping Pathogen-Cellugyrin Interactions
To unravel the molecular intricacies of pathogen-cellugyrin interactions, researchers synthesized cellugyrin peptides representing different regions of the protein. These peptides were then tested for their binding affinity with Cdt subunits and SARS-CoV-2 spike proteins. The findings revealed specific regions within cellugyrin peptides that exhibited preferential binding to both Cdts and SARS-CoV-2 spike proteins, providing insights into the molecular basis of pathogen entry.
Implications for Therapeutic Strategies
The discovery of cellugyrin's role in mediating pathogen entry has profound implications for therapeutic interventions. Modulating cellugyrin expression or disrupting pathogen-cellugyrin interactions could serve as novel strategies to combat a wide range of viral and bacterial infections. These findings pave the way for targeted therapies that interfere with critical cellular entry pathways, ultimately mitigating the impact of infectious diseases.
Future Directions and Research Prospects
While the current study provides significant insights, future research directions aim to delve deeper into the nuances of pathogen-cellugyrin interactions. This includes elucidating the precise mechanisms of binding between pathogens and cellugyrin peptides, exploring the role of other cellular components in these interactions, and assessing the therapeutic potential of targeting cellugyrin-dependent pathways in a clinical setting.
Conclusion: A Unified Understanding of Pathogen Entry
In conclusion, the study represents a significant leap forward in our understanding of pathogen entry mechanisms. By elucidating the shared pathways exploited by both bacterial toxins and viruses, particularly SARS-CoV-2, through cellugyrin-dependent mechanisms, researchers have uncovered a fundamental aspect of host-pathogen interactions. This unified perspective opens doors to innovative therapeutic strategies that target essential cellular entry pathways, offering hope in the ongoing battle against infectious diseases.
The study findings were published in the peer reviewed journal:
Frontiers in Cellular and Infection Microbiology.
https://www.frontiersin.org/articles/10.3389/fcimb.2024.1334224/full
For the Latest
COVID-19 News, keep on logging to Thailand Medical News.
