Study Finds That Epithelial Galectin-3 Induces Mitochondrial Complex Inhibition And Cell Cycle Arrest Of CD8+ T Cells In Severe COVID-19
Thailand Medical News Team Aug 15, 2023 1 year, 8 months, 6 days, 21 hours, 42 minutes ago
COVID-19 Research: The outbreak of SARS-CoV-2 in late 2019 initiated a global pandemic, causing a wide spectrum of clinical manifestations ranging from mild to severe COVID-19 cases. Among the severe cases, CD8+ T cell depletion has emerged as a significant contributor to the disease's progression and poor prognosis. This phenomenon has prompted intensive research efforts to uncover the underlying mechanisms that drive the dysfunction and reduction of CD8+ T cells in severe COVID-19 patients.
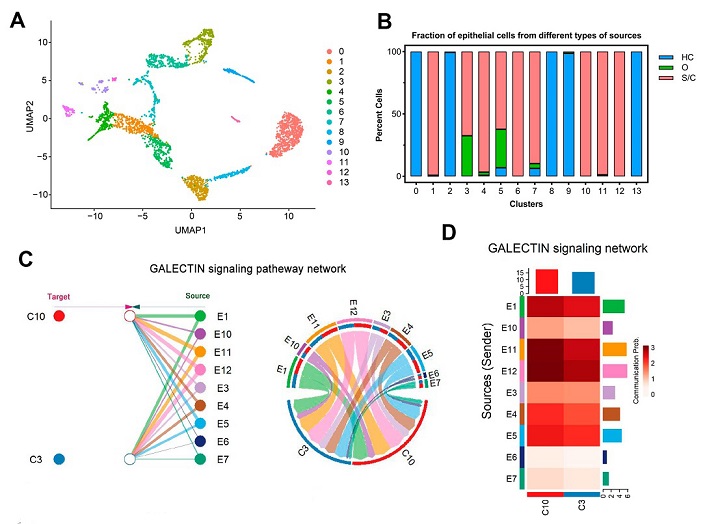 Galectin signaling pathways may be involved in the mitochondrial dysfunction of CD8+ T cells in severe/critical COVID-19 patients. (A) The UMAP presentation of 14 heterogeneous clusters of epithelial cells. (B) Percentage of epithelial cells across healthy controls (HC, blue), moderate (O, green), and severe/critical (S/C, red) COVID-19 patients in individual clusters. (C) Hierarchical plot (left) and chord diagram (right) show the inferred intercellular communication network (only the effect of epithelial cells on CD8+ T cells is represented here) for galectin signaling. E1, 3–7, 10–12: nine epithelial cell groups separated from severe/critical COVID-19 patients; C3, 10: two CD8+ T cell groups from severe/critical COVID-19 patients. Different colors represent different cell clusters. (D) CellChat infers the strength of different cell groups as senders or receivers of signals during cellular communication. The color bar shows the strength of signals, the histograms with different colors indicate the total strength of different cell groups, and the x- and y-axes represent the signal receiver (CD8+ T cells) or sender (epithelial cells).
Galectin signaling pathways may be involved in the mitochondrial dysfunction of CD8+ T cells in severe/critical COVID-19 patients. (A) The UMAP presentation of 14 heterogeneous clusters of epithelial cells. (B) Percentage of epithelial cells across healthy controls (HC, blue), moderate (O, green), and severe/critical (S/C, red) COVID-19 patients in individual clusters. (C) Hierarchical plot (left) and chord diagram (right) show the inferred intercellular communication network (only the effect of epithelial cells on CD8+ T cells is represented here) for galectin signaling. E1, 3–7, 10–12: nine epithelial cell groups separated from severe/critical COVID-19 patients; C3, 10: two CD8+ T cell groups from severe/critical COVID-19 patients. Different colors represent different cell clusters. (D) CellChat infers the strength of different cell groups as senders or receivers of signals during cellular communication. The color bar shows the strength of signals, the histograms with different colors indicate the total strength of different cell groups, and the x- and y-axes represent the signal receiver (CD8+ T cells) or sender (epithelial cells).
In this pursuit, a collaborative study by researchers from Wuhan University of Science and Technology-China and Huazhong University of Science and Technology-China has yielded groundbreaking insights into the immunopathogenesis of severe COVID-19, shedding light on potential therapeutic interventions.
CD8+ T Cell Depletion in Severe COVID-19
The diminished presence of CD8+ T cells in severe COVID-19 patients has been observed and associated with adverse clinical outcomes. Despite these observations, the intricate mechanisms governing this CD8+ T cell reduction remained elusive. Notably, understanding the dynamics of SARS-CoV-2-specific CD8+ T cell responses holds immense promise in devising strategies to combat the virus and mitigate its severe impacts.
Proliferative-Exhausted Phenotype and Impaired Mitochondrial Function
Utilizing cutting-edge single-cell RNA sequencing (scRNA-seq) and single-cell T cell receptor sequencing (scTCR-seq) analyses, the
COVID-19 Research team unveiled a novel proliferative-exhausted CD8+ T cell phenotype in severe/critical COVID-19 patients. Characterized by cell cycle arrest and impaired mitochondrial function, these CD8+ T cells exhibited an aberrant transcriptional profile. Genes associated with respiratory chain complex functions were downregulated, indicating compromised mitochondrial energy production, crucial for CD8+ T cell proliferation and expansion.
The Rol
e of Galectin-3 in CD8+ T Cell Dysfunction
The study delved into the intricate interplay between lung epithelial cells and CD8+ T cells during SARS-CoV-2 infection. Galectin-3, a member of the β-galactoside-binding lectin family, emerged as a key player in CD8+ T cell dysfunction. Upregulated expression of galectin-3, triggered by SARS-CoV-2 ORF3a, was identified as a critical factor contributing to impaired mitochondrial biogenesis and G2/M cell cycle arrest in CD8+ T cells. This revelation marks a significant milestone in understanding the immunopathogenesis of severe COVID-19.
Mechanistic Insights - NRF1 Suppression and Signaling Pathways
Further mechanistic investigations uncovered the role of epithelial galectin-3 in inhibiting the transcription of mitochondrial respiratory chain complex III/IV genes in CD8+ T cells. This inhibition was linked to the suppression of nuclear respiratory factor 1 (NRF1) translocation, a key regulator of mitochondrial gene expression. The study also illuminated the involvement of ERK-related and Akt-related signaling pathways, which contributed to the suppression of NRF1 translocation. Importantly, the galectin-3 inhibitor, TD-139, demonstrated the potential to reverse these effects, thereby enhancing mitochondrial biogenesis and respiratory chain gene expression.
Implications for COVID-19 Treatment
The findings from this study hold significant implications for the development of therapeutic interventions for severe COVID-19 patients. The identification of galectin-3 as a crucial mediator of CD8+ T cell dysfunction provides a novel target for potential pharmacological interventions. TD-139, an existing galectin-3 inhibitor, emerged as a promising candidate to alleviate CD8+ T cell cycle arrest and restore their expansion ability. Furthermore, the study's insights into ERK- and Akt-related signaling pathways offer a deeper understanding of the molecular intricacies underlying CD8+ T cell responses, potentially paving the way for the development of targeted therapies.
Conclusion
The collaborative research conducted by Wuhan University of Science and Technology-China and Huazhong University of Science and Technology-China has unveiled a groundbreaking connection between epithelial galectin-3 expression and CD8+ T cell dysfunction in severe COVID-19. Through comprehensive single-cell analyses and ex vivo experiments, the study has illuminated the mechanisms underlying CD8+ T cell reduction, cell cycle arrest, and impaired mitochondrial function. The identification of galectin-3 as a key modulator offers promising avenues for therapeutic interventions that could alleviate CD8+ T cell dysfunction and improve patient outcomes. By advancing our understanding of the intricate immune responses triggered by SARS-CoV-2, this research has the potential to revolutionize the treatment landscape for severe COVID-19 cases, providing hope for better patient management and outcomes.
The study findings were published in the peer reviewed International Journal of Molecular Sciences.
https://www.mdpi.com/1422-0067/24/16/12780
For the latest
COVID-19 Research, keep on logging to Thailand Medical News.
