Study Finds That Human Serum Albumin Binds To SARS-CoV-2 Spike Protein And Plays A Role In Disease Severity As It Affects The RAS Pathway
Nikhil Prasad Fact checked by:Thailand Medical News Team Apr 03, 2024 1 year, 3 weeks, 2 days, 20 hours, 7 minutes ago
COVID-19 News: The COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2 has brought significant challenges to global public health. As researchers delve deeper into understanding the intricacies of this virus and its impact on human health, they've uncovered intriguing connections between COVID-19 outcomes and factors such as hypoalbuminemia and the renin-angiotensin system (RAS) pathway. In
COVID-19 News report, we will explore the multifaceted role of human serum albumin (HSA) in SARS-CoV-2 infection and disease severity.
 Human Serum Albumin Binds To SARS-CoV-2 Spike Protein
Human Serum Albumin Binds To SARS-CoV-2 Spike Protein
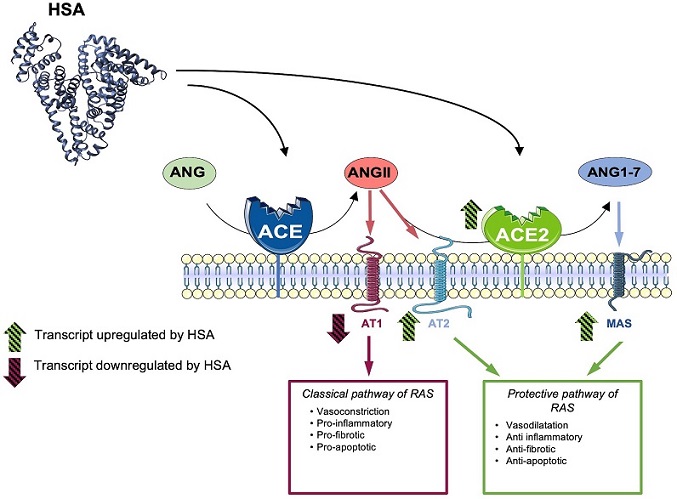
Proposed model for explaining the protective role of HSA towards SARS-CoV-2 infection. A fine balance between the classic and protective pathways of the Renin-Angiotensin System (RAS), under physiological conditions, exists. During SARS-CoV-2 infection, ACE2 is downregulated, leading to an increase in Angiotensin II (Ang II) and Angiotensin 1-7 (Ang 1–7) levels, which promotes the ACE/Ang II/AT2 axis, subsequently triggering a pro-inflammatory and a pro-fibrotic response. In normoalbuminemic individuals, HSA upregulates ACE2, AT2, and MAS, promoting the ACE2/Ang 1–7/AT2-MAS axis. This, in turn, induces anti-inflammatory and anti-fibrotic responses. Therefore, HSA appears to counteract the effects of SARS-CoV-2 on the RAS pathway, contributing to the maintenance of a physiological RAS balance. As a consequence, in hypoalbuminemic patients with COVID-19, the low levels of HSA impede the modulation of the protective arm of RAS. Consequently, the ACE/AngII/AT2 axis triggers the reported pro-inflammatory and pro-fibrotic effects.
SARS-CoV-2 and Human Serum Albumin Interaction: A Molecular Interplay
At the molecular level, the spike (S) protein of SARS-CoV-2 plays a pivotal role in viral entry into host cells. The S protein comprises S1 and S2 subunits, with S1 containing the receptor-binding domain (RBD) crucial for interaction with the host cell receptor angiotensin-converting enzyme 2 (ACE2). ACE2, widely expressed in various organs including the lungs, facilitates viral attachment and entry, making it a prime target for therapeutic interventions.
Studies have highlighted a compelling interaction between HSA and the S1 subunit of the spike protein. HSA, a major protein in human plasma responsible for various physiological functions including maintaining oncotic pressure and transporting essential molecules, exhibits a protective effect against SARS-CoV-2.
In vitro studies using cell cultures infected with SARS-CoV-2 have demonstrated that HSA significantly reduces the viral replication rate. This protective mechanism stems from HSA's ability to bind to the RBD of the spike protein, effectively competing with ACE2 for binding sites. By inhibiting spike protein binding to ACE2, HSA hinders viral attachment and subsequent cellular entry, thus dampening viral replication.
Furthermore, molecular dockin
g studies and displacement-based enzyme-linked immunosorbent assays (ELISA) have corroborated the competitive binding nature of HSA and ACE2 to the spike protein. Despite HSA's relatively lower affinity compared to ACE2, its abundance in plasma compensates for this, contributing to the neutralization of SARS-CoV-2. This interaction underscores the significance of HSA levels in influencing susceptibility to SARS-CoV-2 infection, particularly in individuals with hypoalbuminemia where reduced HSA levels may enhance viral entry and exacerbate disease severity.
Beyond Spike Protein: HSA's Modulation of the Renin-Angiotensin System (RAS) Pathway
In addition to its direct interaction with the spike protein, HSA exerts a profound influence on the renin-angiotensin system (RAS) pathway, a key regulator of blood pressure, electrolyte balance, and fluid homeostasis. ACE2, a critical component of RAS, serves as a counter-regulator by promoting the ACE2/Angiotensin 1-7 (Ang 1-7)/MAS receptor/AT2 axis, often referred to as the "protective arm" of RAS. This axis induces vasodilation, anti-inflammatory responses, and anti-fibrotic effects, counteracting the vasoconstriction and pro-inflammatory effects of the classical RAS pathway.
Studies have revealed that HSA promotes the protective arm of the RAS pathway, aligning its effects with those of angiotensin receptor blockers (ARBs) and ACE inhibitors. By favoring the ACE2/Ang 1-7/MAS receptor/AT2 axis, HSA mitigates acute inflammation, vasoconstriction, and other harmful effects associated with COVID-19. This dual role of HSA in inhibiting viral replication and modulating RAS signaling highlights its potential as a therapeutic target in managing COVID-19 and related complications.
Clinical Implications and Future Directions
The elucidation of HSA's multifaceted role in SARS-CoV-2 infection has significant clinical implications. Firstly, understanding the interplay between HSA levels, viral entry, and disease severity can aid in risk stratification and personalized treatment approaches for COVID-19 patients. Monitoring HSA levels, especially in individuals predisposed to hypoalbuminemia, may offer insights into disease progression and guide therapeutic interventions.
Furthermore, exploring HSA-based therapies or interventions that enhance HSA's protective effects could be a promising avenue in COVID-19 management.
Leveraging HSA's ability to inhibit viral replication and modulate RAS signaling may complement existing treatment strategies and improve patient outcomes, particularly in severe and critical cases.
Future research directions may include:
-Optimizing HSA-based Therapies: Investigating the efficacy and safety of HSA supplementation or HSA-derived products as adjunct therapies in COVID-19 treatment regimens.
-Exploring HSA Variants: Studying genetic variations in HSA that may influence its binding affinity to the spike protein and RAS modulation, potentially leading to personalized therapeutic approaches.
-Long-term Effects: Assessing the long-term impact of HSA levels on COVID-19 recovery, post-acute sequelae, and overall patient prognosis.
-Emerging SARS-CoV-2 Variants: Investigating HSA's efficacy against emerging SARS-CoV-2 variants and potential challenges posed by variant-specific spike protein mutations.
Conclusion
Human serum albumin (HSA) emerges as a multifunctional player in the context of SARS-CoV-2 infection and COVID-19 severity. Its interactions with the spike protein, modulation of the RAS pathway, and potential therapeutic implications underscore the intricate interplay between host factors and viral pathogenesis.
As we navigate through the ongoing COVID-19 pandemic and prepare for future viral threats, further research into HSA's mechanisms of action, clinical applications, and therapeutic potentials is imperative. Integrating HSA-centric approaches into COVID-19 management strategies may offer new avenues for combating the disease and improving patient outcomes in the evolving landscape of infectious diseases.
The study was conducted by researchers from Roma Tre University-Italy, Istituto Zooprofilattico Sperimentale del Mezzogiorno-Italy, Azienda Sanitaria Universitaria Giuliano Isontina (ASUGI)-Italy and Trieste University-Trieste, Italy
The study findings were published in the peer reviewed journal: Aspects of Molecular Medicine.
https://www.sciencedirect.com/science/article/pii/S2949688823000333
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
