Study Finds That Met58 And Di-Acidic Motif Located At C-terminal Region Of SARS-CoV-2 ORF6 Plays A Crucial Role In Its Structural Conformations
Thailand Medical News Team Aug 17, 2023 1 year, 8 months, 5 days, 17 hours, 32 minutes ago
COVID-19 Research: The world has been grappling with the unprecedented challenges posed by the SARS-CoV-2 virus, which has brought global health systems to their knees. Researchers and scientists worldwide have been diligently investigating the intricate molecular details of this virus to unravel its secrets and pave the way for effective countermeasures. In the pursuit of understanding the viral machinery, a team of scientists from the Indian Institute of Technology Mandi has shed light on a hitherto neglected but crucial aspect of the virus - the C-terminal region (CTR) of the ORF6 protein.
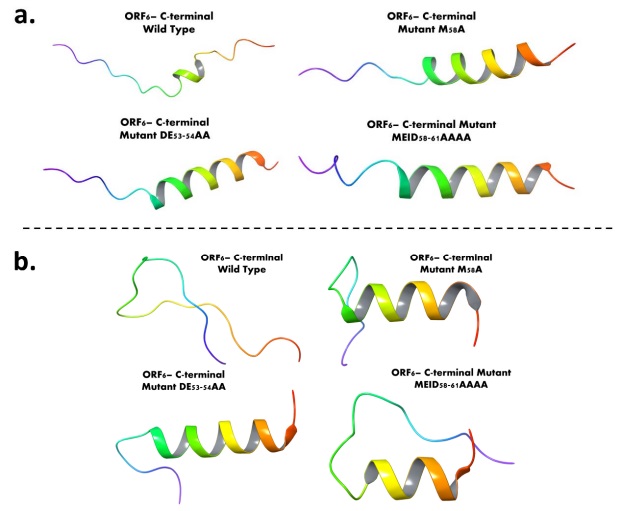 ORF6-CTR wild type and mutant type structures evaluation before and after MD simulations: a. AlphaFold based structure modelling of ORF6-CTR using wild type and mutated sequences (M58A, DE53-54AA, and MEID58-61AAAA). b. The last frames of 500 ns long wild and mutant type structure simulation trajectories
ORF6-CTR wild type and mutant type structures evaluation before and after MD simulations: a. AlphaFold based structure modelling of ORF6-CTR using wild type and mutated sequences (M58A, DE53-54AA, and MEID58-61AAAA). b. The last frames of 500 ns long wild and mutant type structure simulation trajectories
While CTRs have often been overlooked in structural biology, they have emerged as multifunctional elements in both human and viral proteins. These regions, located at the end of a protein's amino acid chain, have been implicated in diverse cellular processes, including protein trafficking, interactions with other proteins and lipids, and cellular localization. Building upon their previous findings concerning the structural dynamics of the SARS-CoV-2 Spike and NSP1 proteins, the study team turned their attention to the CTR (residues 38-61) of the ORF6 protein.
The ORF6 protein is a pivotal player in the SARS-CoV-2 viral arsenal, contributing to viral pathogenesis alongside other structural and non-structural proteins. Although it does not directly impact the virus's structural integrity, ORF6 plays a critical role in evading host immune responses, manipulating host cell metabolic pathways, and creating a conducive environment for viral replication. With its small transmembrane structure, ORF6 disrupts bidirectional transport between the nucleus and cytoplasm, inhibiting the nuclear trafficking of crucial transcription factors and host mRNA.
One of the most intriguing aspects of ORF6 lies in its interaction with the nuclear pore complex components Rae1 and Nup98, orchestrated through its C-terminal region. This interaction acts as a molecular blockade, preventing the docking of cargo import/export protein complexes and further hampering the host's defense mechanisms. The significance of a specific methionine residue at position 58 within the ORF6 C-terminal region has been highlighted in its interaction with the Rae1-Nup98 complex, suggesting its potential as a key molecular mediator in viral pathogenesis.
The
COVID-19 Research team embarked on a comprehensive journey to decipher the structural dynamics of the ORF6 CTR. Employing advanced computational simulations, they investigated various scenarios, including the behavior of the CTR in artificial environments, organic solvents, liposomes, micelle-forming substances, and molecular crowders. This systematic exploration unveiled the intrinsic disorder nature of the ORF6 CTR, except when subjected to high concentrations of organic solvents, where it
demonstrated alpha-helical secondary structures.
The scientists' efforts culminated in the creation of computational models of the ORF6 CTR, both in its wild-type form and with strategically introduced mutations at positions 53, 58, and 61. These models not only validated the experimental observations but also illuminated the integral role of the CTR in structural composition and flexibility. Intriguingly, the mutated sequences exhibited enhanced structural integrity compared to the wild-type structure, hinting at the potential functional implications of these mutations.
Drawing parallels to previous studies on SARS-CoV-2 Spike and NSP1 proteins, the study team postulated that the disordered nature of the ORF6 CTR might facilitate interactions with other viral and host proteins, thereby marking it as a potential target for therapeutic intervention.
Moreover, the interaction between ORF6 and ORF9b, another accessory protein of coronaviruses, gained further significance in light of these findings. It is conceivable that the dynamic interplay between these two proteins hinges on the structural flexibility of the ORF6 CTR.
However, as with any scientific endeavor, this study paves the way for further exploration and experimentation. The study team acknowledge that their speculations require robust in-vitro testing through mutational analyses and interaction studies. By dissecting the molecular intricacies of these interactions, scientists can glean insights into potential therapeutic avenues against SARS-CoV-2.
In conclusion, this new study has illuminated the structural conformations of the ORF6 C-terminal region in SARS-CoV-2. This study not only deepens our understanding of viral pathogenesis but also underscores the critical role of CTRs in modulating protein functions.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2023.08.14.553212v1
For the latest
COVID-19 Research, keep on logging to Thailand Medical News.
