Nikhil Prasad Fact checked by:Thailand Medical News Team Aug 15, 2024 7 months, 4 weeks, 1 day, 6 hours, 20 minutes ago
Coronavirus News: In a groundbreaking study conducted by researchers from the Indian Institute of Technology Kanpur, National Brain Research Centre India, and the German Primate Center, scientists have unveiled a crucial mechanism that enables SARS-CoV-2, the virus responsible for COVID-19, to enter human cells. This discovery sheds light on how the virus exploits specific lipids in our cell membranes, particularly phosphatidylserine (PS), to fuse with and invade host cells. This
Coronavirus News report will delve into the key findings of the study and explore the potential implications for developing new treatments to combat COVID-19.
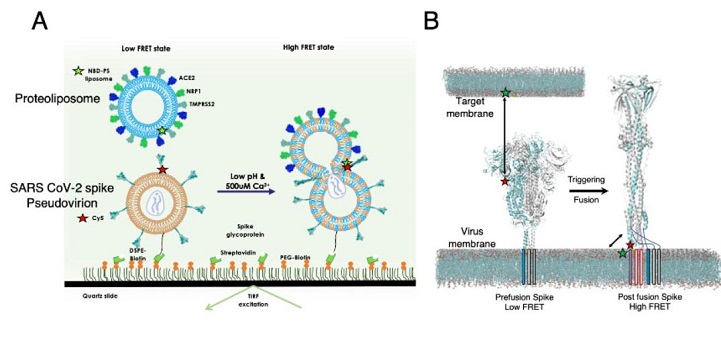 smFRET imaging SARS-CoV-2 spike trimer interaction with PS lipid in target membrane at fusion condition. (A) smFRET imaging assay design for probing the direct interaction between SARS-CoV-2 virus spike glycoprotein and PS lipid in target liposome at fusion condition. The PS lipid is present in the liposome and is labeled with NBD dye (donor) and the spike protein fusion domain is genetically labeled with Cy5 dye (acceptor). The pseudovirion was first immobilized on the quartz slide using biotin-streptavidin linkage, followed by the flowing of liposomes. smFRET imaging was performed at pH 4.6 and pH 4.6 and 500 μM Ca2+ conditions for B.1.617.2 spike and D614G spike pseudovirions. (B) Structural model of the prefusion Delta variants spike trimer with one protomer labeled with acceptor fluorophores (model based on PDB 7SBK; Materials and Methods). Unlabeled protomers within the trimer are shown in gray. The acceptor fluorophore is labeled at the fusion peptide proximal region of S2 (light blue), with the FL residing in a hydrophobic cleft at the protomer interface and contacting the neighboring protomer. The target bilayer membrane has donor fluorophore-labeled PS lipids. If the spike interacts with PS lipid during fusion, the change in FRET is anticipated from low FRET to high FRET state. (C, D) Representative fluorescence (donor, green; acceptor, red) and FRET trajectories (blue) obtained from a single B.1.617.2 spike of pseudovirion during trans interaction with PS lipid of liposome at low pH
Understanding How the Virus Enters Cells
smFRET imaging SARS-CoV-2 spike trimer interaction with PS lipid in target membrane at fusion condition. (A) smFRET imaging assay design for probing the direct interaction between SARS-CoV-2 virus spike glycoprotein and PS lipid in target liposome at fusion condition. The PS lipid is present in the liposome and is labeled with NBD dye (donor) and the spike protein fusion domain is genetically labeled with Cy5 dye (acceptor). The pseudovirion was first immobilized on the quartz slide using biotin-streptavidin linkage, followed by the flowing of liposomes. smFRET imaging was performed at pH 4.6 and pH 4.6 and 500 μM Ca2+ conditions for B.1.617.2 spike and D614G spike pseudovirions. (B) Structural model of the prefusion Delta variants spike trimer with one protomer labeled with acceptor fluorophores (model based on PDB 7SBK; Materials and Methods). Unlabeled protomers within the trimer are shown in gray. The acceptor fluorophore is labeled at the fusion peptide proximal region of S2 (light blue), with the FL residing in a hydrophobic cleft at the protomer interface and contacting the neighboring protomer. The target bilayer membrane has donor fluorophore-labeled PS lipids. If the spike interacts with PS lipid during fusion, the change in FRET is anticipated from low FRET to high FRET state. (C, D) Representative fluorescence (donor, green; acceptor, red) and FRET trajectories (blue) obtained from a single B.1.617.2 spike of pseudovirion during trans interaction with PS lipid of liposome at low pH
Understanding How the Virus Enters Cells
The COVID-19 pandemic has had a devastating impact worldwide, emphasizing the urgent need to understand how the virus operates at a molecular level. At the core of SARS-CoV-2's ability to infect human cells is its spike protein, a structure that protrudes from the virus's surface. This spike protein acts as a molecular machine, facilitating the fusion between the viral membrane and the host cell membrane, which is a critical step for the virus to deliver its genetic material into the cell and begin the infection process.
Researchers have long known that the spike protein interacts with the angiotensin-converting enzyme 2 (ACE2) receptor on the surface of human cells. However, the exact mechanism by which the spike protein triggers membrane fusion and what role, if any, specific lipids in the host cell membrane play in this pro
cess has remained unclear.
The Role of Phosphatidylserine Lipid
In their study, the researchers utilized a combination of steady-state membrane fusion assays and advanced imaging techniques to investigate how the spike protein interacts with lipids in the host cell membrane. They discovered that phosphatidylserine (PS), a lipid commonly found on the inner leaflet of the cell membrane, plays a crucial role in mediating membrane fusion.
When the spike protein comes into contact with phosphatidylserine (PS) on the surface of the target cell, it triggers a series of conformational changes that bring the viral and host membranes into close proximity, facilitating fusion. This interaction is further enhanced by low pH conditions and the presence of calcium ions (Ca2+), which are known to induce conformational changes in the spike protein.
Key Findings of the Study
One of the most significant findings of the study is that the interaction between the spike protein and phosphatidylserine (PS) is not limited to a single variant of SARS-CoV-2. The researchers observed that this interaction is conserved across all major variants of concern, including D614G, Alpha, Beta, Delta, and Omicron. This suggests that PS is a universal target for the virus, regardless of its specific mutations.
Furthermore, the study demonstrated that when phosphatidylserine (PS) is sequestered or absent from the host cell membrane, the spike-mediated fusion process is significantly impaired, leading to a reduction in viral infectivity. This finding opens up new possibilities for therapeutic interventions aimed at blocking this critical step in the viral entry process.
Implications for COVID-19 Interventions
The discovery that phosphatidylserine (PS) is indispensable for SARS-CoV-2 spike-mediated fusion has profound implications for the development of new treatments and preventative measures against COVID-19. By targeting the interaction between the spike protein and PS, it may be possible to design drugs or therapies that effectively block the virus from entering cells.
One potential approach could involve the use of molecules that mimic phosphatidylserine (PS), binding to the spike protein and preventing it from interacting with the actual PS lipids in the host cell membrane. Alternatively, therapies that reduce the externalization of PS on the cell surface could be explored as a means of inhibiting viral entry.
The Broader Impact of Lipid-Mediated Fusion
The study also contributes to our broader understanding of how enveloped viruses, such as SARS-CoV-2, exploit host cell lipids to facilitate membrane fusion. The role of lipids in viral entry has been observed in other viruses, such as HIV and Ebola, suggesting that lipid-mediated fusion could be a common strategy employed by many different pathogens.
By further elucidating the mechanisms behind lipid-mediated fusion, scientists can develop more effective antiviral therapies that target these processes, potentially providing a new line of defense against a wide range of viral infections.
Conclusion
In conclusion, this study provides critical insights into the molecular mechanisms that enable SARS-CoV-2 to enter human cells. The discovery that the spike protein relies on interactions with phosphatidylserine lipids to trigger membrane fusion opens up new avenues for therapeutic interventions aimed at blocking this essential step in the viral life cycle. As the COVID-19 pandemic continues to evolve, these findings could play a crucial role in shaping the development of new treatments and preventative measures.
The study findings were published in the peer-reviewed journal: mBio.
https://journals.asm.org/doi/10.1128/mbio.01077-24
For the latest
Coronavirus News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/study-reveals-shifts-in-key-inflammatory-markers-known-as-adipokines-in-covid-19-patients
https://www.thailandmedical.news/news/sars-cov-2-uses-histone-deacetylase-6-to-boost-its-own-replication
