Study Finds That RNA Virus-Derived Components Are Able To Regulate Of cGAS-STING Signaling
Nikhil Prasad Fact checked by:Thailand Medical News Team May 03, 2024 10 months, 4 weeks, 2 days, 18 hours, 22 minutes ago
Medical News: The realm of infectious diseases is constantly evolving, with RNA viruses standing out as significant contributors to global health challenges. These viruses, including influenza, hepatitis C, and the notorious SARS-CoV-2, continue to pose substantial threats due to their ability to mutate rapidly and evade immune surveillance. As we delve into the complexities of RNA virus infections, it becomes clear that understanding the interplay between these pathogens and the host immune system is paramount for developing effective therapeutic strategies. A new study by researchers from Zhejiang University School of Medicine-China and Shangyu People’s Hospital of Shaoxing-China that is covered in this
Medical News report found that RNA virus-derived components are able to regulate of cGAS-STING signaling.
 RNA Virus-Derived Components Are Able To Regulate Of cGAS-STING Signaling
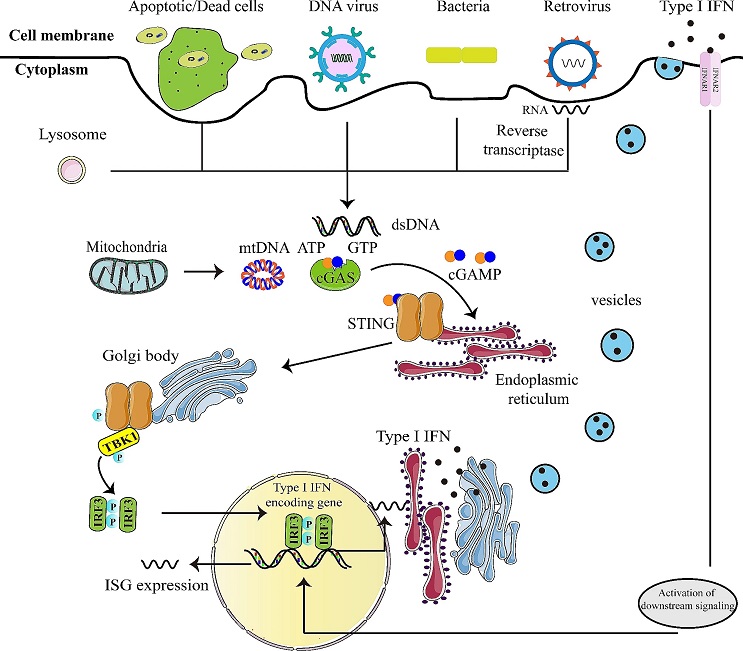
Schematic diagram of cGAS-STING signaling pathway. (cGAS, as an innate immune sensor, is able to recognize various cytoplasmic dsDNA from pathogens, apoptotic/dead cells, mitochondria, and others. The interaction between cGAS and dsDNA leads to enzymatic activation of cGAS and catalyzing the formation of 2’,3’-cyclic GMP-AMP (cGAMP) from ATP and GTP. cGAMP binds to the dimer of interferon gene stimulatory factor (STING) located on the endoplasmic reticulum (ER) membrane. Then, STING moves from the ER to Golgi body via the ER–Golgi intermediate compartment, and then serves as a signaling platform for TBK1 phosphorylation. TBK1 phosphorylates the C-terminal domains of STING, and then IRF3 is recruited and phosphorylated. Finally, dimerized IRF3 can act as a transcription factor to initiate the transcription of type-I IFN and subsequent induction of ISG expression, eliciting antiviral defense)
The Clinical Spectrum of RNA Virus Infections: A Multifaceted Challenge
RNA Virus-Derived Components Are Able To Regulate Of cGAS-STING Signaling
Schematic diagram of cGAS-STING signaling pathway. (cGAS, as an innate immune sensor, is able to recognize various cytoplasmic dsDNA from pathogens, apoptotic/dead cells, mitochondria, and others. The interaction between cGAS and dsDNA leads to enzymatic activation of cGAS and catalyzing the formation of 2’,3’-cyclic GMP-AMP (cGAMP) from ATP and GTP. cGAMP binds to the dimer of interferon gene stimulatory factor (STING) located on the endoplasmic reticulum (ER) membrane. Then, STING moves from the ER to Golgi body via the ER–Golgi intermediate compartment, and then serves as a signaling platform for TBK1 phosphorylation. TBK1 phosphorylates the C-terminal domains of STING, and then IRF3 is recruited and phosphorylated. Finally, dimerized IRF3 can act as a transcription factor to initiate the transcription of type-I IFN and subsequent induction of ISG expression, eliciting antiviral defense)
The Clinical Spectrum of RNA Virus Infections: A Multifaceted Challenge
RNA viruses encompass a diverse array of pathogens responsible for a spectrum of clinical manifestations. From mild respiratory infections to life-threatening diseases, these viruses can wreak havoc on human health. The ongoing COVID-19 pandemic, caused by SARS-CoV-2, exemplifies the clinical diversity and challenges posed by RNA viruses. While many infections result in mild symptoms, severe cases can lead to acute respiratory distress syndrome (ARDS), multi-organ failure, and death.
The cGAS-STING Signaling Pathway: A Key Player in Innate Immunity
Central to the innate immune response against viral infections is the cGAS-STING signaling pathway. This intricate cascade serves as a sentinel for aberrant DNA, triggering a robust antiviral response characterized by the production of type I interferons (IFN-I) and other immune mediators. The pathway's core components, including cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING), orchestrate a coordinated defense against viral invaders.
RN
A Virus-Derived Components and Their Impact on cGAS-STING Signaling
-SARS-CoV-2 Interactions with cGAS-STING: The complex interactions between SARS-CoV-2 and the cGAS-STING pathway highlight the virus's ability to both activate and evade immune responses. While viral components such as the spike protein can induce IFN-I production through cGAS-STING activation, other viral proteins like ORF10 and PLP counteract this response, leading to immune suppression and disease progression.
-HIV-1: Subverting Immune Surveillance: Human immunodeficiency virus type 1 (HIV-1) employs various strategies to manipulate cGAS-STING signaling, promoting viral replication and immune evasion. Proteins like viral infectivity factor (Vif) facilitate the recruitment of inhibitory factors like SHP-1 to dampen STING-mediated antiviral responses, contributing to HIV persistence and pathogenesis.
-HCV: Fine-Tuning Immune Responses: Hepatitis C virus (HCV) demonstrates a delicate balance between activating and inhibiting cGAS-STING signaling. While STING activation can limit HCV replication, viral proteins like NS4B antagonize this pathway, allowing the virus to evade immune detection and establish chronic infection.
-ZIKV and DENV: Modulating Host Defenses: Zika virus (ZIKV) and dengue virus (DENV) showcase how RNA viruses strategically manipulate cGAS-STING signaling to facilitate viral replication. Protease-mediated degradation of cGAS or STING, as seen in DENV infection, highlights the intricate mechanisms viruses employ to subvert host immune defenses.
-Broader Perspectives: Insights from Influenza and Other RNA Viruses: Beyond specific examples, other RNA viruses like influenza (IAV), encephalomyocarditis virus (EMCV), and lymphocytic choriomeningitis virus (LCMV) also engage with cGAS-STING signaling in nuanced ways. These interactions influence the balance between antiviral immunity and viral persistence, shaping disease outcomes.
Therapeutic Implications and Future Directions in Targeting cGAS-STING Signaling
The dynamic interplay between RNA viruses and the cGAS-STING pathway presents intriguing therapeutic opportunities. Strategies aimed at modulating cGAS-STING signaling hold promise for combating viral infections. For instance, STING agonists have shown efficacy in limiting viral replication and enhancing antiviral immune responses. Conversely, inhibitors targeting viral components that suppress cGAS-STING activation offer alternative approaches to disrupt viral immune evasion strategies.
The Road Ahead: Advancing Understanding and Innovation in Antiviral Therapies
As research advances, a deeper understanding of the intricate mechanisms governing cGAS-STING signaling regulation by RNA virus-derived components is essential. Exploring the dual role of this pathway in both promoting antiviral immunity and potentially exacerbating inflammatory responses underscores the need for precision in therapeutic targeting. The development of novel therapeutics, including STING agonists, viral protease inhibitors, and immunomodulatory agents, represents a promising frontier in antiviral drug discovery.
Conclusion: A Holistic Approach to Combatting RNA Virus Infections
In conclusion, unraveling the intricacies of cGAS-STING signaling regulation by RNA virus-derived components offers a holistic perspective on antiviral immunity. From understanding the molecular mechanisms of viral evasion to leveraging host immune responses for therapeutic benefit, the intersection of virology and immunology presents exciting avenues for innovation. Moving forward, interdisciplinary research efforts and strategic therapeutic interventions will be instrumental in combating the ever-evolving landscape of RNA virus infections and improving global public health outcomes.
The study findings were published in the peer reviewed Virology Journal (Springer Link).
https://link.springer.com/article/10.1186/s12985-024-02359-1
For the latest
Medical News, keep on logging to Thailand Medical News.
