Study Finds That SARS-CoV-2 Nsp15 Suppresses Type I Interferon Production By Inhibiting IRF3 Phosphorylation And Nuclear Translocation
Thailand Medical News Team Aug 29, 2023 2 years, 3 months, 3 weeks, 4 days, 6 hours, 44 minutes ago
COVID-19 Research: The year 2019 marked the emergence of a novel coronavirus, SARS-CoV-2, setting in motion a global pandemic that continues to challenge public health systems and economies worldwide. Responsible for the coronavirus disease 2019 (COVID-19), this virus belongs to the family Coronaviridae, genus Betacoronavirus, and has garnered immense attention due to its rapid transmission and severe clinical outcomes. Unraveling the strategies employed by SARS-CoV-2 to subvert the host immune system is paramount for devising effective therapeutic interventions and preventive measures. One such strategy involves the viral protein Nsp15, which has been implicated in suppressing the production of interferons (IFNs), key components of the host's innate immune response.
 Graphical Abstract
The SARS-CoV-2 Arsenal: Evasion of Innate Immunity
Graphical Abstract
The SARS-CoV-2 Arsenal: Evasion of Innate Immunity
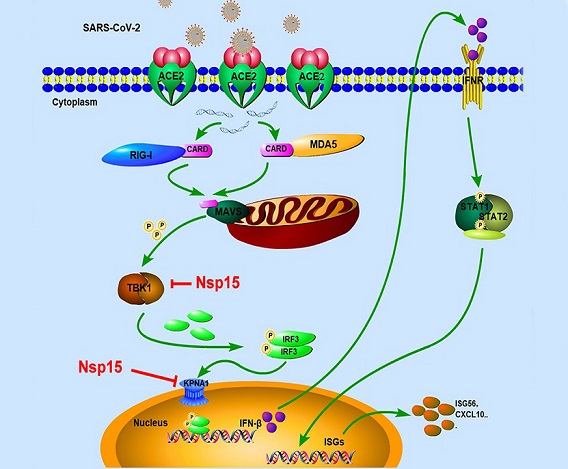
The battle between SARS-CoV-2 and the host's immune system is an intricate interaction where the virus seeks to establish infection and evade detection, while the host strives to neutralize and eliminate the invader. A pivotal role in this conflict is played by the host's innate immune system, which acts as the first line of defense against viral infections. At the forefront of this defense are type I interferons (IFNs), signaling proteins that orchestrate a cascade of antiviral responses upon viral invasion. These responses are triggered by the recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs), such as the retinoic-inducible gene-I (RIG-I)-like receptors (RLRs).
Nsp15: A Multi-Faceted Viral Player
Among the viral proteins implicated in manipulating the host immune response, Nsp15 has emerged as a significant antagonist of the type I IFN response.
Understanding the mechanisms underlying Nsp15's actions is crucial for deciphering the molecular intricacies of SARS-CoV-2-host interactions. Nsp15, also known as EndoU, is a poly-U-specific endonuclease that plays a pivotal role in the viral replication-transcriptase complex (RTC). By cleaving viral RNA sequences containing 5'-Poly U, Nsp15 assists the virus in evading detection by the host's innate immune sensors, a strategy observed in other coronaviruses as well.
A Dual-Pronged Suppression Strategy
This new
COVID-19 Research by scientists from the Affiliated Taizhou People's Hospital of Nanjing Medical University-China, Jiangsu University-Zhenjiang-China and the Affiliated Yixing Hospital of Jiangsu University-China has provided valuable insights into the intricate ways by which Nsp15 interferes with the host's immune response.
&
lt;br />
The study identified two distinct mechanisms through which Nsp15 suppresses IFN production:
-Disruption of TBK1-IRF3 Interaction: In the cascade leading to IFN production, TBK1 and IRF3 play pivotal roles. Nsp15 was found to competitively interact with TBK1, a crucial kinase in the RLR signaling pathway. This interaction impedes the binding of TBK1 to IRF3, inhibiting IRF3's phosphorylation. Phosphorylated IRF3 is central to the transcription of IFN genes, and its disruption by Nsp15 thus curbs the production of IFNs.
-Targeting Nuclear Translocation: Phosphorylated IRF3 must translocate to the nucleus for initiating IFN production. Nsp15, in a groundbreaking discovery, was found to interact with karyopherin α1 (KPNA1), a protein responsible for the nuclear import of IRF3. This interaction results in the degradation of KPNA1 through autophagy, effectively preventing the nuclear translocation of phosphorylated IRF3 and further dampening the host's antiviral responses.
Implications and Future Directions
The findings from this study offer a profound understanding of how SARS-CoV-2 exploits the host's immune response machinery to its advantage. Nsp15's multifaceted approach in suppressing IFN production highlights the virus's adaptability and sophistication in thwarting the host's defense mechanisms. Furthermore, the study underscores the importance of elucidating the interplay between viral proteins and host factors for a comprehensive understanding of COVID-19 pathogenesis.
Future research avenues could involve exploring the role of Nsp15's endoribonuclease activity in modulating IFN responses. Additionally, investigating the mechanisms that lead to KPNA1 autophagy-dependent degradation and identifying the autophagy receptors involved in this process could provide a more comprehensive picture of Nsp15's suppression strategy.
In conclusion, the study's insights into the actions of Nsp15 shed light on the intricate strategies SARS-CoV-2 employs to evade the host's immune surveillance. Unraveling these mechanisms not only deepens our understanding of COVID-19 but also opens up potential avenues for developing targeted therapeutic interventions.
The study findings were published in the peer reviewed journal: iScience.
https://www.cell.com/iscience/fulltext/S2589-0042(23)01782-0
For the latest
COVID-19 Research, keep on logging to Thailand Medical News.
