Source: Multiple Jul 21, 2018 7 years, 6 months, 2 weeks, 4 days, 4 hours, 48 minutes ago
Scientists from UNSW Sydney and the UK have discovered that the human immunodeficiency virus (HIV) hijacks a small molecule from the host cell to protect itself from being destroyed by the host's immune system.
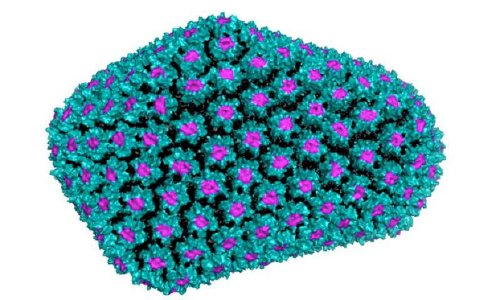
Inositol hexakisphosphate fits into the pore structures (pink) of the HIV capsid (teal), and makes the makes the capsid stronger, protecting the genetic material inside. Credit: UNSW
The finding, as well as details of the new strategy that enabled it, are published as back-to-back papers in eLife. They identify a new target for antiviral therapy
against HIV and provide a method for testing and measuring
new drugs designed to target the capsid.
UNSW Ph.D. student Chantal Márquez is involved in both studies and is the first author of the paper describing the new method.
HIV forms a protein shell—called a capsid—that shields its genetic material from host defence mechanisms as it enters the cell and makes its way to the nucleus to establish infection.
Using a new single-molecule microscopy technique—developed at UNSW's Single Molecule Science in the Faculty of Medicine—the research teams found that HIV specifically incorporates a small molecule from the host cell—inositol hexakisphosphate—to strengthen its capsid. The host inadvertently provides the key for the virus infecting it to lock down the protective shell, keeping the genetic cargo safe until it is released into the nucleus.
"The HIV capsid falls apart within minutes once it's isolated from the virus," said Associate Professor Till Böcking, who led the UNSW team involved in both studies.
"Our strategy lets us study exactly how a native capsid breaks apart in real-time without taking it out of the viral membrane."
With the help of Associate Professor Stuart Turville of the Kirby Institute, the team engineered viruses with fluorescent tags to monitor the viral capsid using fluorescence microscopy.
"We can now see the effect of different
molecules on the capsid, and pinpoint precisely when it cracks open and begins to collapse," says Associate Professor Böcking.
"Capsids need to be much more stable inside a cell because the infection process takes hours, not minutes—so we wanted to find out what keeps it stable inside a cell," says Dr. David Jacques of Single Molecule Science, who is an author of both studies.
The researchers found that inositol hexakisphosphate, which is abundantly present inside mammalian
cells, makes the capsid much stronger, stabilizing it for 10-20 hours.
"It's like a switch. When you bind this molecule, you stabilize the capsid, and release the molecule to open it up," explains Associate Professor Böcking.
"The H
IV capsid has been intensively studied, but the question of how it can simultaneously be both stable and poised to 'uncoat' has been one of the great unanswered questions in HIV biology," says Dr. Leo James, leader of the research team at the Medical Research Council Laboratory of Molecular Biology in Cambridge, UK.
Most of the currently approved HIV therapies target enzymes needed at different stages of the virus' life cycle, but none of them are directed at the HIV capsid. New drug alternatives could improve the treatment of HIV with reduced toxic effects.
Reference: University Of new South Wales, Donna L Mallery et al, IP6 is an HIV pocket factor that prevents capsid collapse and promotes DNA synthesis,
eLife (2018). DOI: 10.7554/eLife.35335