Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 06, 2024 1 year, 4 weeks, 6 hours, 54 minutes ago
COVID-19 News: The emergence of the novel coronavirus, SARS-CoV-2, has led to a global health crisis, impacting individuals with various health predispositions differentially. Beyond its primary effects on the respiratory system, there is growing concern about its potential to affect neurological health, particularly in individuals with neurodegenerative diseases. Recent studies have suggested a possible link between SARS-CoV-2 infection and the progression of conditions such as Parkinson's disease (PD) and Alzheimer's disease (AD). Understanding the mechanisms underlying these interactions is crucial for devising effective preventive and therapeutic strategies.
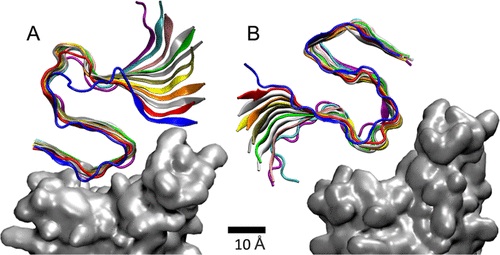 SARS-CoV-2 Can Bind To Amyloid Beta Fibrils.
MD simulations of (A) nonpolar and (B) acidic sides (residues) of the Aβ42 (12-mer) fibril coupled to RBD in the spike of SARS-CoV-2 (Omicron).
SARS-CoV-2 Can Bind To Amyloid Beta Fibrils.
MD simulations of (A) nonpolar and (B) acidic sides (residues) of the Aβ42 (12-mer) fibril coupled to RBD in the spike of SARS-CoV-2 (Omicron).
In a groundbreaking study conducted jointly by the University of Illinois at Chicago, USA, and the University of Haifa, Israel that is covered in this
COVID-19 News report, researchers investigated the direct interaction between SARS-CoV-2 and amyloid beta (Aβ) fibrils, which are implicated in neurodegenerative diseases. Utilizing molecular dynamics simulations, the study aimed to elucidate the potential binding of SARS-CoV-2 spike proteins to Aβ fibrils, shedding light on the mechanisms underlying neurological complications associated with COVID-19.
Background
SARS-CoV-2 gains entry into human cells by binding its spike proteins to angiotensin-converting enzyme 2 (ACE2) receptors present on cell membranes. The mutations observed in the spike protein, particularly in the Omicron variant, have been associated with enhanced binding affinity to host cell receptors.
Moreover, evidence suggests that SARS-CoV-2 may exploit alternative pathways to enter the brain, potentially influencing the progression of neurodegenerative diseases.
Previous observations, including temporary improvements in communication abilities in PD patients following COVID-19 infection, have sparked interest in exploring the relationship between SARS-CoV-2 and neurodegenerative disorders. Studies have demonstrated the ability of SARS-CoV-2 to bind to amyloid proteins such as αsynuclein and influence their aggregation. Additionally, viruses like SARS-CoV-2 can generate peptides that self-assemble in brain tissues, potentially exacerbating neuronal damage.
Results and Discussion
The molecular dynamics simulations conducted in this study revealed several stable binding configurations of Aβ42 fibrils to the spike proteins of both Omicron and Alpha variants of SARS-CoV-2. By analyzing the interactions between specific residues of Aβ42 fibrils and the receptor binding domains (RBDs) of SARS-CoV-2 spike proteins, the researchers identified distinct b
inding patterns and dynamics.
The simulations demonstrated that Aβ42 fibrils could bind to both Omicron and Alpha RBDs, with varying degrees of stability. The nonpolar side of the fibril exhibited stronger binding affinity, primarily mediated by van der Waals interactions. However, the charged side of the fibril also formed stable interactions, albeit to a lesser extent.
Moreover, the study observed dynamic sliding movements of the fibrils on the RBD surfaces, indicating a potential mechanism for viral interference with neurological processes. The differential binding preferences of Aβ42 fibrils to Omicron and Alpha RBDs suggest complex interactions between the viral spike proteins and amyloid structures, which may have implications for disease progression and therapeutic interventions.
Implications and Future Directions
The findings of this study provide valuable insights into the molecular mechanisms underlying the interaction between SARS-CoV-2 and amyloid beta fibrils, with potential implications for neurological health. Understanding how the virus interacts with amyloid proteins could inform the development of novel therapeutic strategies targeting neurodegenerative diseases and COVID-19 complications.
Future research directions may include experimental validation of the predicted binding configurations and characterization of the structural and functional consequences of these interactions in neuronal cell models. Additionally, investigations into the potential role of amyloid fibrils as reservoirs for viral persistence and dissemination in the central nervous system could uncover new avenues for therapeutic intervention.
In conclusion, the study highlights the intricate interplay between viral pathogens and neurodegenerative processes, underscoring the importance of multidisciplinary approaches in elucidating the complexities of COVID-19-related neurological complications. By unraveling the molecular underpinnings of these interactions, researchers can pave the way for targeted interventions aimed at mitigating the impact of SARS-CoV-2 on neurological health.
The study findings were published in the peer reviewed journal: American Chemical Society.
https://pubs.acs.org/doi/10.1021/acsomega.3c08481
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
