Study Shows That Transmembrane Domains Play an Important Role in BST-2 And SARS-CoV-2 ORF7a Interactions
SARS-CoV-2 Research - BST-2- ORF7a May 23, 2023 1 year, 10 months, 4 weeks, 3 hours, 57 minutes ago
SARS-CoV-2 Research: The ongoing COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has highlighted the need for a better understanding of the interactions between the virus and host proteins. In particular, the role of accessory proteins, such as ORF7a, in modulating host responses is of great interest.
 Graphical Abstract
Graphical Abstract
A recent
SARS-CoV-2 Research conducted by scientists from the University of Virginia and the University of Maryland College Park aimed to investigate the structural basis for the interactions between the human protein BST-2 (also known as Bone marrow stromal antigen 2, tetherin or CD317) and SARS-CoV-2 ORF7a, with a specific focus on the involvement of transmembrane domains. The findings of this study shed light on the importance of transmembrane domains in regulating the interactions between BST-2 and ORF7a and provide insights into the mechanisms by which these interactions may influence viral pathogenesis.
Understanding the Role of ORF7a in SARS-CoV-2 Infection
SARS-CoV-2 is the virus responsible for the global COVID-19 pandemic. It enters host cells through the binding of its spike protein to the angiotensin-converting enzyme 2 (ACE2) receptor. The viral genome contains various open reading frames (ORFs), including the ORF7a, which encodes a transmembrane protein. Previous studies have shown that ORF7a can suppress the interferon response and induce the expression of proinflammatory cytokines. However, the molecular details of how ORF7a interacts with host proteins and contributes to viral pathogenesis are not fully understood.
BST-2: A Key Player in Antiviral Defense
BST-2 is an antiviral protein that plays a crucial role in blocking the release of enveloped viruses from infected cells. It functions by tethering the viral particles to the host cell membrane, preventing their spread. BST-2 has been shown to inhibit the release of various viruses, including HIV-1 and human coronaviruses. However, viruses have also developed strategies to counteract BST-2's antiviral activity by interacting with specific domains of the protein.
Investigating BST-2 ORF7a Interactions
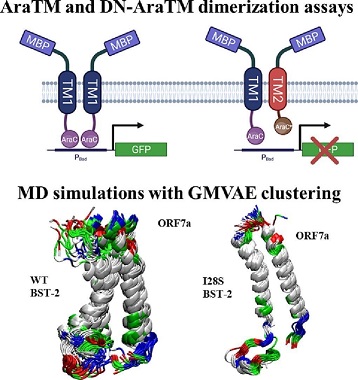
In this study, the research team aimed to elucidate the specific interactions between BST-2 and SARS-CoV-2 ORF7a, with a particular focus on the role of transmembrane domains. They conducted experiments using dimerization assays and molecular dynamics simulations to understand the structural basis of these interactions.
The results of the study showed that the transmembrane domains of BST-2 and ORF7a are crucial for their interactions. Mutations in the transmembrane domain of BST-2, such as the I28S variant, altered the heterodimerization between BST-2 and ORF7a. Molecular dynamics simulations provided insights into the protein-protein interface and revealed that the I28S mutation enhanced helix-helix hydrophobic interactions, promo
ting a strong helix-packing in the heterodimeric configuration.
Furthermore, the study team observed that SARS-CoV-2 ORF7a disrupted the glycosylation of BST-2. This disruption of glycosylation is consistent with previous studies and suggests a mechanism by which ORF7a can antagonize BST-2's antiviral activity.
Implications of the Study
Overall, the study provides valuable insights into the role of transmembrane domains in BST-2 ORF7a interactions and their impact on BST-2 function. The findings highlight the importance of these interactions in modulating viral pathogenesis and suggest that mutations within the transmembrane domain of BST-2 can alter its susceptibility to ORF7a-mediated antagonism. Further research in this area could contribute to the development of targeted interventions that disrupt these interactions and potentially inhibit viral replication.
As the COVID-19 pandemic continues to pose a global health threat, understanding the intricate interactions between SARS-CoV-2 and host factors is of utmost importance. Studies like this contribute to our knowledge of the molecular mechanisms underlying viral infection and can pave the way for the development of novel therapeutic strategies to combat the virus.
The study findings were published in the peer reviewed journal: Biochimica et Biophysica Acta (BBA) – Biomembranes.
https://www.sciencedirect.com/science/article/pii/S0005273623000561
For the latest
SARS-CoV-2 Research, keep on logging to Thailand Medical News.
