Study Unveils Details Of SARS-CoV-2 Stealthy Assault On The Heart And Finds That Captopril Can Be Used As A Therapeutic Agent
COVID-19 Research - Heart - Captopril May 21, 2023 1 year, 10 months, 4 weeks, 10 hours, 21 minutes ago
COVID-19 Research: In a significant stride towards understanding the cross-organ effects of SARS-CoV-2, the virus behind the COVID-19 pandemic, a collaborative team of scientists from prestigious Chinese institutes have uncovered fascinating insights as well as revealing the mechanism behind the virus's assault on our hearts. Their study offers hope in the form of a potential therapeutic strategy.
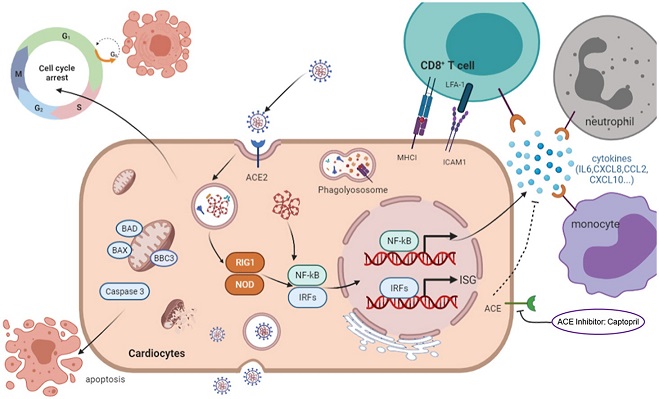 Graphical Abstract
Graphical Abstract
Up to this point, COVID-19 has primarily been perceived as a respiratory disease, but as the crisis unfolds, a different picture is emerging. It turns out, about 20% of patients suffer from cardiac complications. Moreover, the prognosis for patients with pre-existing cardiovascular disease who contract COVID-19 is significantly poorer.
Researchers from the Chinese Academy of Sciences in Guangzhou, University of Chinese Academy of Sciences in Beijing, and The First Affiliated Hospital of Guangzhou Medical University have delved into the mystery of SARS-CoV-2's cardiac impact. Their investigation has identified some crucial mechanisms at play and hints at a promising course of treatment with a drug called Captopril, an ACE inhibitor typically used for hypertension management.
Their exploratory study utilized a non-transgenic mouse model infected with the Beta variant (B.1.351) of SARS-CoV-2. Interestingly, the viral RNA was detectable not just in the lungs, but also in the hearts of these mice. Pathological analyses showed disrupted and ruptured myocardial fibers, thinner ventricular walls, and mild inflammation and fibrosis in the hearts of the infected mice.
Moreover, the study demonstrated that SARS-CoV-2 could infect cardiomyocytes, the cells making up our heart muscles, and incite apoptosis - programmed cell death. Infected cells were observed to suffer from reduced mitochondrial integrity and quantity, which translated into a cessation of beating in these cells. This phenomenon was found in human pluripotent stem cell-derived cardiomyocyte-like cells (hPSC-CMs), an excellent model to study cardiac diseases in vitro.
The
COVID-19 Research team also performed a comprehensive transcriptome sequencing analysis at different time points post-infection. This technique allowed the team to create an intricate molecular timeline of the infection, showing robust inflammatory responses, upregulation of MHC class I molecules, and activation of apoptosis signaling. All these processes collectively seem to amplify inflammation, immune cell infiltration, and cell death, resulting in severe heart damage.
To connect these dots to a potential therapy, the team introduced Captopril to their experimental system. Surprisingly, this common hypertension drug demonstrated potent abilities to soothe the inflammation
and apoptosis incited by the SARS-CoV-2 infection. It seemed to achieve this by inactivating the TNF signaling pathways, pivotal in driving inflammation and cell death.
It's worth noting that ACE inhibitors have been previously suggested to potentially increase the risk of severe COVID-19 due to their effect on ACE2 levels. However, this research, consistent with recent meta-analyses, suggests that this might not be the case. The mechanism through which Captopril counteracts the potential increase in viral entry caused by increased ACE2 remains to be thoroughly explored, but it appears that it could be related to Captopril's inhibitory effect on ACE2 shedding.
This new data offers an intriguing explanation for the cardiac injury experienced by COVID-19 patients. However, the results also underscore the complexity of this viral disease. While SARS-CoV-2 primarily invades the respiratory system, the insidious secondary cardiac involvement is increasingly apparent.
The virus uses a protein called angiotensin converting enzyme 2 (ACE2) as an entry point into cells. This protein is abundantly present in the cells lining our airways but is also found on cardiomyocytes. As it turns out, patients with cardiovascular diseases tend to have even higher ACE2 levels than healthy individuals, making their hearts more susceptible to viral attack.
Upon infection, SARS-CoV-2 can trigger cell death or apoptosis and disrupt cell growth in cardiomyocytes. In addition, the virus induces a potent antiviral response, releasing a deluge of inflammatory mediators that facilitate the infiltration of immune cells, leading to tissue damage. Notably, the virus's assault can also result in severe alterations in cardiac structure and function.
One of the most compelling findings was that SARS-CoV-2 infection leads to a notable down-regulation of crucial sarcomeric structural proteins such as titin (TTN) and Nebulin (NEB). This down-regulation can critically impact the contraction capacity of the heart at both cellular and tissue levels. In other words, the heart's ability to pump effectively is reduced, leading to signs and symptoms of heart failure.
A closer look at the infected cardiomyocytes revealed that the virus wreaks havoc on the cellular level as well. Infected cardiomyocytes showed an alarming decrease in mitochondrial integrity and quantity, leading to energy depletion. Mitochondria, often referred to as the "powerhouses of the cell," play a critical role in the production of cellular energy. With their integrity compromised, the energy production required for the cardiomyocytes to function optimally dwindles significantly. This impacts the myocardium, the heart muscle responsible for the beating of the heart, leading to a reduction in the beating rate of the heart and possible arrhythmias.
Another surprising aspect of the SARS-CoV-2 infection was its impact on the cell cycle, causing cell cycle arrest. In simple terms, the normal process of cell growth and division was put on hold. This was deduced from observing the expression levels of several key genes associated with cell cycle regulation. Genes like CDK1, CDK6, CCNE2, and others that ordinarily help in regulating the cell cycle showed a decrease in infected cardiomyocytes. The effect of this interruption in the cell cycle can lead to abnormal growth and cell death.
In essence, this groundbreaking research has thrown light on how SARS-CoV-2 can directly infect the heart, induce heart cell damage, and trigger systemic inflammation, contributing to the dire cardiac complications observed in COVID-19 patients. More importantly, it suggests that drugs like Captopril could potentially help mitigate these severe complications, especially in patients with underlying cardiovascular conditions.
The study findings were published in the peer reviewed journal: Antiviral Research.
https://www.sciencedirect.com/science/article/pii/S0166354223001146
For the latest
COVID-19 Research, keep on logging to Thailand Medical News.
