Study Warns Of Rare Occurrence Of Individuals Developing Stage III Hypertension After mRNA-Based COVID-19 Vaccines!
Source:Hypertension And mRNA Vaccines Sep 18, 2021 3 years, 7 months, 1 week, 5 days, 4 hours, 45 minutes ago
A study by researchers from Lausanne University Hospital-Switzerland has found that an extremely small percentage of otherwise healthy individuals who receive the mRNA vaccines such as those by Pfizer or Moderna, run the risk of developing stage III hypertension upon vaccination.
The study findings were published in the peer reviewed journal: Hypertension (A Journal of the American Heart Association)
https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.121.17316
The vaccination center in Lausanne in Western Switzerland, a city of 140 000 inhabitants started the COVID-19 vaccination with mRNA-based vaccines on Januray 11
th 2021.
By the 9
th of February, 13 296 vaccine doses were administered with 12 349 patients receiving the first dose (10501 Pfizer/BioNTech, 1848 Moderna) and 947 receiving a second dose (945 Pfizer/BioNTech, 2 Moderna). The center offers vital signs monitoring for any patient reporting symptoms compatible with a serious adverse event such as suspicion of anaphylactic reaction, malaise, shortness of breath, or pain (eg, headache and chest pain). Any adverse event is medically monitored and those which are serious or unexpected are reported to Swissmedic ie the Swiss Agency for Therapeutic Products.
The study team reported a case series of 9 patients with stage III hypertension documented within minutes of vaccination during the first 30 days, of which 8 were symptomatic.
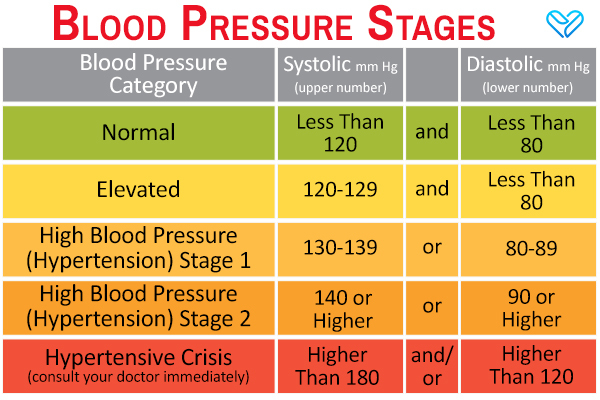
Stage-three-hypertension is actually referred to as “stage 1.” Systolic is between 140 and 159 mm Hg and diastolic is between 90 and 99 mm Hg. A person at stage 1 is in danger of a variety of health complications due to moderate hypertension. At this point, a person would be expected to try medication to reduce their blood pressure and risk of heart disease and/or stroke. These drugs include ACE inhibitors, angiotensin receptor blockers, beta blockers, thiazide diuretics, and calcium channel blockers. They would also be expected to make diet and lifestyle changes.
Vital signs were measured with an oscillometric manometer (Omron Healthcare Europe; a HEM 907-E7) with at least 3 sets of separate values at 5-minute intervals. Median age was 73 (IQR, 22) years and sex distribution was 7 women for 2 men. Eight of 9 patients had a history of arterial hypertension with most patients on antihypertensive therapy. All but one patient received the Pfizer/BioNTech (BNT162b2) vaccine. Of note, the Moderna (mRNA-1273) vaccine was only introduced in late January in Switzerland. One of the patients (n=3) reported a cerebral aneurysm that was coiled within the last year, with a targeted SBP <140 mm Hg.
1 Due to developing headache, the patient underwent imaging with no sign of intracranial hemorrhage. Patient No. 4 did not have associated ECG changes or an increase in hs-troponins. Importantly, all patients recovered but required at most several hours of monitoring at our tertiary center’s emergency department.
Due to the patient high turnover in the vaccination center, the center did not have prevaccination BP values. However, 8 of 9 patients reported otherwise well controlled hyper
tension.
The case series suggests that a fraction of hypertensive patients may react with symptomatically significant increases in both systolic and diastolic blood pressure. A stress response is likely in view of the public debate, in addition to pain response and white coat effect ie the latter being associated with age and female sex.
However, the relatively low heart rate (median, 73 bpm) may soften this hypothesis. Alternative mechanisms could theoretically include hypertension to components of the vaccines such as polyethylenglycol, although this seems unlikely due to the presumably low dosage and as patients reacted within minutes of the injection. As tromethamine is only contained in the MRNA-1273 vaccine, its causative role is ruled out.
An interaction between the S-protein and angiotensin converting enzyme 2 also seems highly unlikely as patients reacted within minutes of the injection, not leaving time for mRNA cellular uptake, translation, and S-protein presentation at the membrane of macrophages and dendritic cells.
It must be noted that hypertension has not been mentioned explicitly as an adverse event in both safety/immunogenicity trials.
More data are needed to understand the extent and the mechanism of hypertension after mRNA-based vaccination.
The study findings indicate that in elderly patients with a history of hypertension or significant prior cardiovascular comorbidities, prevaccination control of blood pressure and post-vaccination monitoring, including symptom screening may be warranted.
Another case was also documented in the Journal of the American Academy of Allergy, Asthma and Immunology.
https://www.aaaai.org/allergist-resources/ask-the-expert/answers/2021/hypertension
In that case, a 42-year-old woman received her first dose of Moderna vaccine. After 30 minutes while walking to her car, she became flushed and tachycardia. This continued and BP was elevated up to 180/130.
Many similar cases are being reported across the world and individuals should refrain from immediately going back to work or even driving immediately after having a mRNA vaccine. Instead their conditions should be properly monitored for at least 24 hours with proper rest required prior to resuming normal routines.
For more on
mRNA Vaccines, keep on logging to Thailand Medical News.

