Swiss Study Finds That Influenza A Viruses Exploit Transferrin Receptor Recycling For Entry Into Host Cells
Nikhil Prasad Fact checked by:Thailand Medical News Team May 05, 2024 1 year, 7 months, 2 weeks, 5 days, 11 hours, 53 minutes ago
Influenza News: The perpetual battle between viruses and their host cells is a captivating saga in the realm of virology. Among the vast array of viruses, the Influenza A virus (IAV) stands out for its ability to swiftly adapt and infect a diverse range of hosts, including humans and animals. While considerable knowledge exists regarding IAV's initial attachment to host cells via sialic acids, the precise mechanisms governing its entry into cells have remained enigmatic. Recent groundbreaking research from the University of Geneva in Switzerland that is covered in this
Influenza News report has shone a new light on this aspect, particularly focusing on the intricate role of transferrin receptor 1 (TfR1) recycling in facilitating IAV entry.
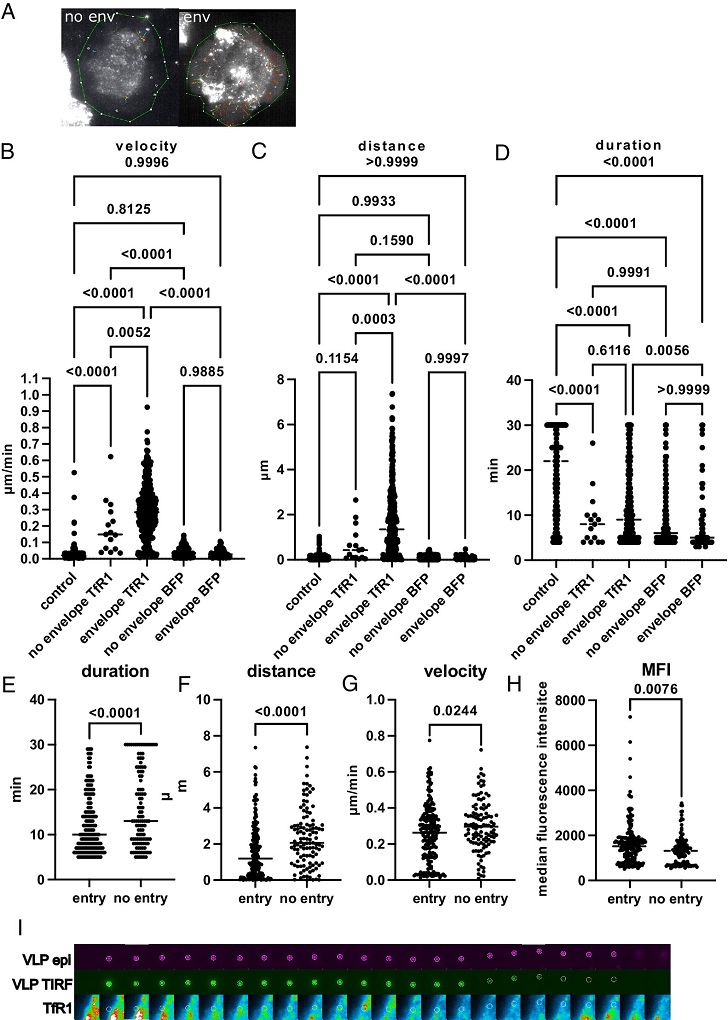 Influenza A Viruses Exploit Transferrin Receptor Recycling For Entry Into Host Cells. TfR1 colocalizes with entering IAV. CHO Pro5 TfR1 BFP or CHO Pro5 BFP were incubated with mNeonGreenM1 VLP and monitored for 30 min with a TIRF microscope. (A) Visualization of tracks recorded with HA/NA enveloped VLP or non enveloped VLP. Representative cells are shown. Detected VLP is indicated with a white circle. Tracks are color-coded for the starting time point (blue: beginning of Movie S1, red: end of it). (B–D) Comparison of track duration in min (B), track distance in µm (C), and mean VLP velocity in µm/min (D) for control tracks (HA/NA enveloped VLPs outside of the cell surface area, n = 269), tracks of non enveloped VLP (lacking HA/NA, n = 15) on the cell surface of TfR1 expressing cells, tracks of HA/NA enveloped VLP on the cell surface of TfR1 expressing cells (n = 371), tracks of non enveloped VLP (lacking HA/NA, n = 254) on the cell surface of BFP expressing cells and tracks of HA/NA enveloped VLP on the cell surface of BFP expressing cells (n = 66). Each dot represents a single track. Control tracks and enveloped VLP tracks on cell surfaces were recorded from three cells in three independent experiments, tracks from nonenveloped viruses were recorded from three cells in one experiment. Median values are indicated by a line, P values were calculated with one way ANOVA. (E–G) Tracks from enveloped VLPs on cells organized into entering VLPs (TIRF signal lost before frame 30, n = 199) and non entering VLPs (TIRF signal remains until frame 30, n = 113). Track duration (E), track distance (F) and VLP velocity (G) are indicated as for B–D, with the respective median indicated by a line. P values were calculated by t test. (H) Median fluorescence intensity of TfR1-BFP along the tracks of VLPs that enter or do not enter until frame 30. Each dot represents a single data point of individual tracks. Only tracks of six or more frames were considered. (I) Example of an enveloped VLP entering the host cell. The VLP is tracked in TIRF and epifluorescence mode.
The Quest for Entry Receptors
Influenza A Viruses Exploit Transferrin Receptor Recycling For Entry Into Host Cells. TfR1 colocalizes with entering IAV. CHO Pro5 TfR1 BFP or CHO Pro5 BFP were incubated with mNeonGreenM1 VLP and monitored for 30 min with a TIRF microscope. (A) Visualization of tracks recorded with HA/NA enveloped VLP or non enveloped VLP. Representative cells are shown. Detected VLP is indicated with a white circle. Tracks are color-coded for the starting time point (blue: beginning of Movie S1, red: end of it). (B–D) Comparison of track duration in min (B), track distance in µm (C), and mean VLP velocity in µm/min (D) for control tracks (HA/NA enveloped VLPs outside of the cell surface area, n = 269), tracks of non enveloped VLP (lacking HA/NA, n = 15) on the cell surface of TfR1 expressing cells, tracks of HA/NA enveloped VLP on the cell surface of TfR1 expressing cells (n = 371), tracks of non enveloped VLP (lacking HA/NA, n = 254) on the cell surface of BFP expressing cells and tracks of HA/NA enveloped VLP on the cell surface of BFP expressing cells (n = 66). Each dot represents a single track. Control tracks and enveloped VLP tracks on cell surfaces were recorded from three cells in three independent experiments, tracks from nonenveloped viruses were recorded from three cells in one experiment. Median values are indicated by a line, P values were calculated with one way ANOVA. (E–G) Tracks from enveloped VLPs on cells organized into entering VLPs (TIRF signal lost before frame 30, n = 199) and non entering VLPs (TIRF signal remains until frame 30, n = 113). Track duration (E), track distance (F) and VLP velocity (G) are indicated as for B–D, with the respective median indicated by a line. P values were calculated by t test. (H) Median fluorescence intensity of TfR1-BFP along the tracks of VLPs that enter or do not enter until frame 30. Each dot represents a single data point of individual tracks. Only tracks of six or more frames were considered. (I) Example of an enveloped VLP entering the host cell. The VLP is tracked in TIRF and epifluorescence mode.
The Quest for Entry Receptors
Viruses employ a myriad of strategies to penetrate host cell defenses and establish infection. A pivotal phase in this process is the attachment and subsequ
ent entry into host cells, often mediated by specific protein-protein interactions between viral ligands and host receptors. For IAV, the initial attachment occurs through its major surface glycoprotein hemagglutinin (HA) binding to sialic acids present on host cell surfaces. However, this initial attachment is merely the first step, as viral entry necessitates interactions with specific entry receptors.
Unraveling the Role of Transferrin Receptor 1 (TfR1)
The cutting-edge study conducted by the Swiss researchers employed innovative techniques to unravel the mysteries surrounding IAV entry. Proximity ligation coupled with mass spectrometry identified TfR1 as a promising candidate entry protein for IAV. TfR1, a type II transmembrane protein primarily involved in iron uptake, is known to undergo continuous recycling between the cell surface and endosomes. Intriguingly, the researchers discovered that TfR1 recycling plays a crucial role in facilitating IAV entry, thereby unveiling a fascinating "revolving door" mechanism exploited by the virus.
Functional Insights from Genetic and Pharmacological Studies
Further delving into the functional aspects, genetic knockout experiments targeting TfR1 in cell lines yielded compelling results. The absence of TfR1 led to a significant impairment in early viral replication, underscoring its pivotal role as an entry factor for IAV. Conversely, experiments involving the overexpression of human TfR1 demonstrated a marked enhancement in viral entry, highlighting the critical importance of TfR1 levels in determining host susceptibility to IAV infection.
Expanding upon these findings, the researchers delved into pharmacological interventions targeting TfR1. The use of ferristatin II, a chemical inhibitor of TfR1, resulted in a substantial reduction in viral entry and subsequent replication in vitro. This pharmacological inhibition not only validated TfR1 as a potential therapeutic target for combating IAV but also hinted at its broader implications in antiviral drug development.
Insights into Viral-Host Interactions
Further investigations into the molecular interactions between IAV and TfR1 revealed intriguing insights. While direct binding of IAV particles to TfR1 was observed, this interaction was not strictly necessary for viral entry. This discovery led to the exploration of alternative mechanisms, including the potential role of TfR1 in promoting virion uptake in trans, possibly through interactions with other cell surface proteins. Notably, experiments involving a "headless" mutant of TfR1 lacking its extracellular domain still facilitated IAV entry, highlighting the multifaceted nature of TfR1's involvement in viral uptake pathways.
Implications for Antiviral Development
The implications of these findings extend far beyond fundamental virology research. The identification of TfR1 as a key player in IAV entry opens avenues for the development of novel antiviral therapies. Ferristatin II, by selectively interfering with TfR1 function, demonstrated promising results in limiting IAV entry and subsequent replication both in vitro and in vivo. This approach not only holds potential for mitigating IAV infections but also paves the way for exploring similar strategies against other viruses that exploit TfR1-mediated endocytosis for cellular entry.
Conclusion
In conclusion, the groundbreaking research provides a comprehensive understanding of how IAV strategically leverages TfR1 recycling mechanisms for efficient entry into host cells. By deciphering these intricate viral-host interactions, the study not only enhances our fundamental knowledge of viral entry pathways but also lays the groundwork for targeted antiviral strategies. The discovery of TfR1 as a potential therapeutic target signifies a significant step forward in the ongoing battle against influenza and other viral infections, heralding a new era of precision antiviral therapies with broader implications for public health and beyond.
The study findings were published in the peer reviewed journal: Proceedings of The National Academy of Sciences (PNAS).
https://www.pnas.org/doi/10.1073/pnas.2214936120
For the latest
Influenza News, keep on logging to Thailand Medical News.
