Taiwanese Study Finds Methylglyoxal as the Culprit Causing Retinopathy in Diabetics
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 05, 2024 5 months, 1 week, 1 day, 9 hours, 59 minutes ago
Medical News: Researchers from Taipei Medical University, Cardinal Tien Hospital-Taiwan, and National Taiwan University have unveiled new insights into the role of methylglyoxal (MGO) in diabetic retinopathy, a major cause of vision loss worldwide. MGO, a reactive byproduct of glucose metabolism, has been pinpointed as a key contributor to the damage seen in the retinas of diabetic individuals. Their findings reveal how MGO affects the retina's cells, setting the stage for potential new treatments.
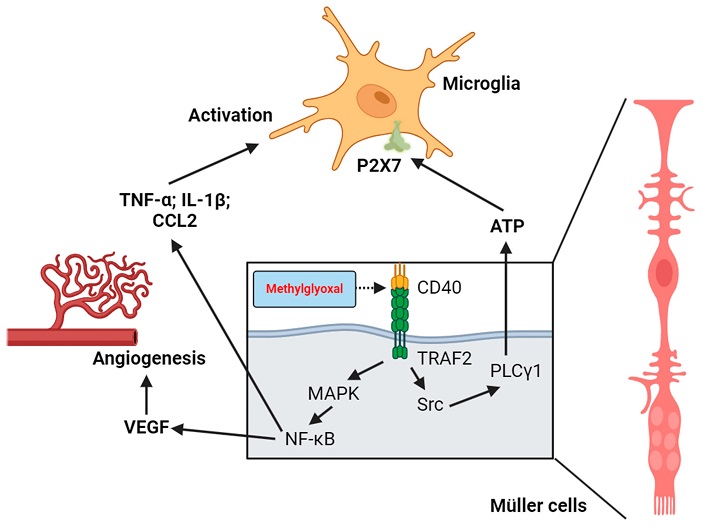 Roles of CD40 activation and Müller cell–microglia communication in MGO-induced chronic inflammation and promotion of new blood vessel formation in the retina.
Roles of CD40 activation and Müller cell–microglia communication in MGO-induced chronic inflammation and promotion of new blood vessel formation in the retina.
This
Medical News report explains that diabetic retinopathy, a progressive disease affecting the retina's blood vessels, often develops in people who have lived with diabetes for over 15 years. The condition causes inflammation, oxidative stress, and eventually loss of vision. Elevated blood sugar levels accelerate the production of MGO, which forms compounds known as advanced glycation end products (AGEs). These AGEs accumulate in the retina, impacting cell function and exacerbating retinal damage.
Understanding Diabetic Retinopathy
Diabetic retinopathy (DR) is a condition that affects the retina’s blood vessels and is one of the leading causes of vision impairment. DR progresses through multiple stages, starting with mild changes in blood vessels to severe non-proliferative DR and eventually leading to proliferative DR, where new blood vessels form abnormally. These new vessels can cause retinal detachment, bleeding, and permanent vision loss. The team studied how MGO and AGEs influence these changes in retinal cells and contribute to DR’s progression.
How MGO Affects the Retina
MGO is highly reactive and readily modifies proteins and DNA through a process called glycation, a non-enzymatic reaction that alters cell function. Glycated proteins become AGEs, which activate the receptor for AGEs (RAGE) in retinal cells. The study observed how MGO impacts various cellular pathways, causing damage to retinal pigment epithelium (RPE) cells, Müller cells (support cells for retinal neurons), microglia (immune cells of the retina), and pericytes (cells that support blood vessels). In the retina, this results in chronic inflammation and a higher risk of retinal cell death.
Key Mechanisms of MGO's Impact on Retinal Cells
-Glycation of Proteins: MGO modifies proteins in the retina, creating AGEs that damage critical proteins like crystallins, albumin, and low-density lipoprotein (LDL). For example, MGO’s glycation of crystallins affects protein structure, decreasing the eye’s transparency and contributing to vision impairment. MGO also glycosylates albumin, leading to cell death in the retina’s pericytes and disrupting the blood-retinal barrier.
-Oxidative Stress and Inflammation: The presence of AGEs amplifies oxidative stress wit
hin retinal cells, creating a cycle of cellular damage. AGEs raise intracellular levels of reactive oxygen species (ROS), promoting inflammation, which accelerates retinal cell deterioration. Oxidative stress was notably higher in cells with MGO exposure, indicating a potential target for antioxidant treatments to protect retinal health.
-Activation of the RAGE Pathway: When MGO-modified proteins bind to the RAGE receptor in retinal cells, it triggers inflammation and accelerates damage in diabetes-prone retinal tissue. This study showed that MGO-RAGE interactions also increase ROS levels and contribute to oxidative stress, which is particularly damaging to RPE and Müller cells. These cells play key roles in retinal health, and their dysfunction leads to worsening diabetic retinopathy.
Exploring Cell-Specific Impacts
Each cell type within the retina is affected differently by MGO:
-Retinal Pigment Epithelium (RPE) Cells: MGO glycates crystallin proteins in RPE cells, weakening the cells' ability to prevent apoptosis (cell death) and increasing cell dysfunction.
-Müller Cells: Serving as the retina's support cells, Müller cells experience inflammation due to MGO-triggered RAGE pathway activation. This inflammation further activates microglia, causing more cell damage.
-Retinal Microglia: Microglia become hyperactive in the presence of MGO, worsening inflammation, disrupting the blood-retinal barrier, and making retinal tissue more susceptible to bleeding and edema.
-Pericytes and Endothelial Cells: MGO induces oxidative stress in these cells, contributing to diabetic complications like abnormal blood vessel growth, commonly seen in the later stages of diabetic retinopathy.
Detoxifying MGO to Protect Retinal Cells
The body has a natural defense against MGO through a detoxification system involving glyoxalase enzymes, mainly glyoxalase-1 (Glo1) and glyoxalase-2 (Glo2). This study highlights how MGO levels rise in diabetics due to decreased glyoxalase activity, which exacerbates MGO’s effects on retinal cells. Findings suggest that boosting glyoxalase activity in the retina could provide a protective effect, helping manage the damaging accumulation of MGO. Research shows that certain natural compounds, like those that activate the Nrf2 pathway, may promote glyoxalase function and enhance the body's ability to clear MGO.
Potential Treatments to Counteract MGO Damage
The researchers note promising strategies for preventing and treating diabetic retinopathy, focusing on the pathways affected by MGO. Antioxidants, anti-inflammatory drugs, and compounds that enhance glyoxalase activity are under exploration. Additionally, inhibiting the RAGE pathway could be a way to limit inflammation and cellular damage in the retina. Several treatments targeting these pathways are currently under review, with the goal of creating therapies that protect against MGO-induced damage in diabetic retinopathy.
A Promising Avenue for Future Research
The study underlines the need for advanced diagnostic tools, including artificial intelligence, to detect DR early and improve treatment outcomes. By identifying MGO's role in DR, researchers hope to find new treatments that could slow or prevent the progression of this vision-threatening condition. Clinical trials testing new antioxidants, glyoxalase inducers, and RAGE inhibitors are among the next steps. The study suggests a potential benefit in combining these treatments with regular monitoring of retinal health, especially in patients with diabetes.
Conclusion
This groundbreaking study reveals the significant role of MGO in the development of diabetic retinopathy, a condition that affects millions of people worldwide. By promoting glycation, oxidative stress, and inflammation in the retina, MGO accelerates the progression of diabetic retinopathy and leads to vision loss. The research suggests that treatments targeting MGO’s damaging effects and enhancing the glyoxalase detoxification pathway may help in managing this disease. Further investigation into natural compounds that activate Nrf2 and reduce MGO's impact could also offer valuable therapeutic options.
The study findings were published in the peer-reviewed journal: Biomedicines.
https://www.mdpi.com/2227-9059/12/11/2512
For the latest Diabetic Retinopathy, keep logging on to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/natural-compounds-from-green-tea-and-coleus-forskohlii-show-promise-for-glaucoma-and-diabetic-retinopathy
https://www.thailandmedical.news/news/the-role-of-microrna-in-diabetic-retinopathy
