TF inhibition With A Novel COVID-19 Thromboprophylaxis: rNAPc2 Safe But Did Not Reduce D-Dimer Levels More Than Heparin Standard Of Care
Thailand Medical News Team Aug 22, 2023 1 year, 8 months, 10 hours, 44 minutes ago
COVID-19 Thromboprophylaxis: The global healthcare community has been grappling with the multifaceted challenges posed by the COVID-19 pandemic, driven by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). One of the most perplexing aspects of this viral infection has been the heightened risk of thrombo-inflammation, a phenomenon that has led to grave complications and even death in many cases. Among the various components of this complex coagulopathy, tissue factor (TF) emerges as a central player, driving disordered coagulation and inflammation in viral infections. As researchers tirelessly search for new therapeutic avenues to combat COVID-19, the potential of rNAPc2, a novel tissue factor inhibitor, has been investigated in the ASPEN-COVID-19 Trial.
https://classic.clinicaltrials.gov/ct2/show/NCT04655586
Graphical Abstract
Understanding the Need for Novel Therapies
The onset of the COVID-19 pandemic thrust the world into a medical crisis of unparalleled proportions. Early studies revealed a troubling propensity for thrombosis in COVID-19 patients, encompassing a range of thrombotic events from microthrombi to arterial and venous thromboses. This heightened coagulability, often marked by elevated D-dimer levels, was not effectively addressed by conventional anticoagulant therapies. This apparent failure of existing treatments underscores the need for innovative approaches that target the unique thrombo-inflammatory dynamics of COVID-19.
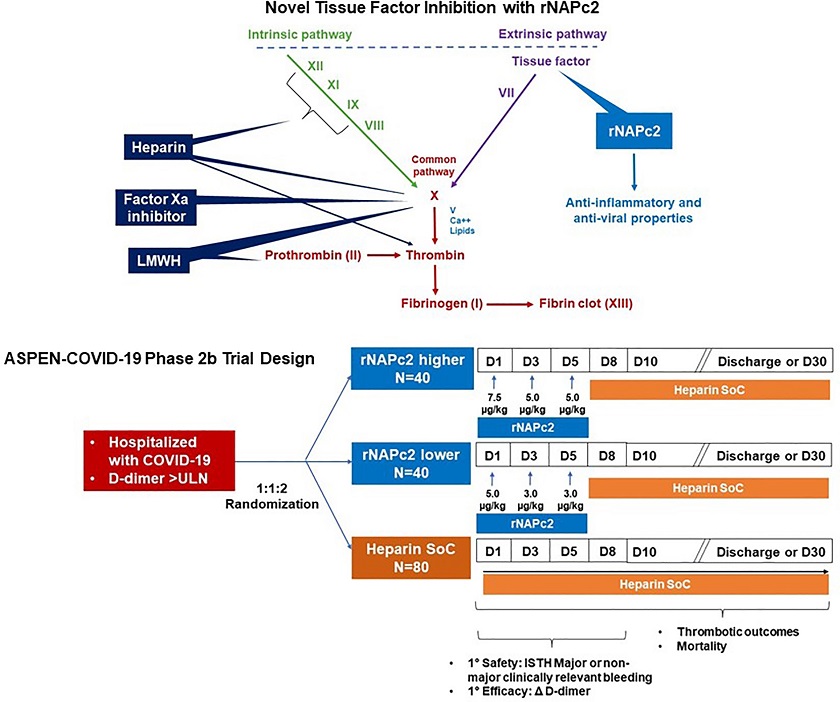
Introducing rNAPc2: A Novel Therapeutic Candidate
Enter rNAPc2, a recombinant nematode anticoagulation protein c2. Derived from the saliva of the hookworm Ancyclostoma caninum, this protein exhibits potent tissue factor inhibitory properties, making it a promising candidate for mitigating the coagulopathy associated with COVID-19. rNAPc2's intricate mode of action involves binding to factor X and subsequently to the factor VIIa/TF catalytic complex, thereby inhibiting the extrinsic coagulation pathway.
Additionally, rNAPc2's potential to modify tissue factor signaling in viral infections introduces a novel dimension - its role in antiviral immunity. This dual function renders rNAPc2 an intriguing prospect for managing the intricate thrombo-inflammatory landscape of COVID-19.
ASPEN-COVID-19 Trial Design and Methodology
The ASPEN-COVID-19 Trial was a phase 2b international, multicenter endeavor
COVID-19 Thromboprophylaxis study aimed at eval
uating the safety and efficacy of rNAPc2 compared to heparin in COVID-19 patients at elevated thrombotic risk. Hospitalized patients were randomly assigned to receive either rNAPc2 or heparin, with their respective outcomes meticulously assessed. Safety was the primary endpoint, with bleeding events and other adverse effects closely monitored. The secondary endpoint focused on efficacy, specifically the change in D-dimer concentration from baseline to day 8 or discharge if before day 8.
Primary Findings: Safety and Efficacy Evaluation
Among the 160 patients who participated in the trial, rNAPc2 treatment emerged as well-tolerated, devoid of excess bleeding or serious adverse events. This encouraging safety profile lends credibility to the potential clinical utility of rNAPc2. However, in terms of efficacy, the trial's primary endpoint - change in D-dimer levels - did not show significant differences between rNAPc2 and heparin-treated patients. This particular outcome needs to be interpreted cautiously, considering the variability in D-dimer levels and the potential influence of other COVID-19 therapies.
Unveiling Patterns: Severity-Based Analysis
The variability in patient response to treatment was further explored in a severity-based analysis. Among patients with severe disease, both rNAPc2 and heparin failed to significantly reduce D-dimer levels. However, in patients with milder illness, rNAPc2 demonstrated a nearly twofold greater reduction in D-dimer compared to heparin, although statistical significance wasn't achieved. This intriguing pattern suggests that rNAPc2 may have a more pronounced effect among less severely affected patients, mirroring observations made with heparin.
Implications and Considerations
Despite the limitations of the trial, including its relatively small size and changes in background therapies, the ASPEN-COVID-19 study has generated critical insights. Its results shed light on rNAPc2's safety and potential efficacy, laying the foundation for further investigation into its use in conditions where tissue factor-driven pathophysiology is implicated. This includes cancer-associated thrombosis, cytokine storms associated with oncological therapies, and conditions marked by recurrent venous thromboembolism.
The Road Ahead: A Multifaceted Approach
As the world continues to grapple with the ever-evolving challenges posed by COVID-19, the ASPEN-COVID-19 trial provides a valuable stepping stone in the journey to better understand and combat the thrombo-inflammatory dynamics of the disease. The notion of combining rNAPc2 with heparin - a strategy supported by the trial's observations - opens up new possibilities for a multifaceted therapeutic approach. Future studies will need to explore rNAPc2's impact on longer-term outcomes, consider its potential in conjunction with evolving treatment strategies, and investigate its broader applicability in other diseases characterized by tissue factor-driven pathophysiology.
In conclusion, the ASPEN-COVID-19 trial has illuminated the potential of rNAPc2 in addressing the thrombo-inflammatory challenges of COVID-19. While the trial's findings reveal limitations and underscore the complexities of this global health crisis, they also inspire hope for innovative therapeutic solutions that can navigate the intricate interplay between coagulation, inflammation, and viral infection.
The findings of the ASPEN-COVID-19 trial were published in the peer reviewed journal: Arteriosclerosis, Thrombosis and Vascular Biology (AHA Journal)
https://www.ahajournals.org/doi/10.1161/ATVBAHA.122.318748
For more about
COVID-19 Thromboprophylaxis, keep on logging to Thailand Medical News.
