Thapsigargin And Tunicamycin Block SARS-CoV-2 Cell Entry Via Differential Modulation Of Unfolded Protein Response, AKT Signaling And Apoptosis
Nikhil Prasad Fact checked by:Thailand Medical News Team May 04, 2024 11 months, 3 weeks, 2 days, 45 minutes ago
COVID-19 News: The ongoing COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2 has spurred intense research efforts worldwide to understand its pathogenesis and identify potential therapeutic strategies. Among the many areas of investigation, the role of endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) in SARS-CoV-2 infection has emerged as a significant focus. This
COVID-19 News delves into a detailed exploration of how acute pre-existing ER stress, induced by Thapsigargin (THA) and Tunicamycin (TUN), impacts SARS-CoV-2 cell entry and subsequent host cell responses.
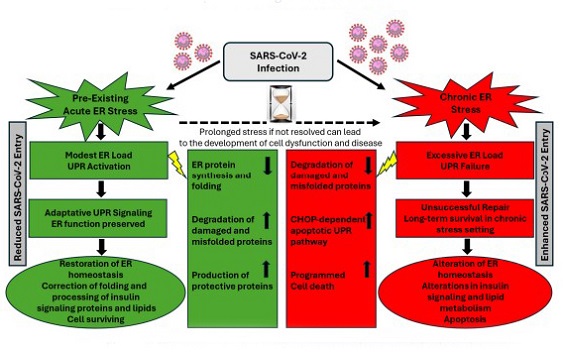 The status of cellular ER stress may play a crucial role in determining whether stressed cells will survive SARS-CoV-2 infection or undergo programmed cell death. The transition from pre-existing acute stress to chronic stress following SARS-CoV-2 infection is a complex process that can vary depending on specific circumstances such as the nature of stressors, duration of stress, and the capacity of infected host cells to cope with the stress. Activation of UPR during a pre-existing ER stress may represent an early host defense mechanism to restore normal cell function and ER homeostasis by promoting the correct folding and processing of proteins and lipids, ultimately leading to reduced SARS-CoV-2 entry into host cells. If the stress is not resolved quickly and remains persistent and prolonged due to UPR hyperactivity and inadequate cellular repair mechanisms, stressed cells become more prone to virus infection, which can cause an aggravated pathological chronic ER stress-associated cellular dysfunction, ultimately affecting the clustering and localization of viral entry receptors on the cell surface, impairing SARS-Co-V-2 entry into host cells.
Understanding ER Stress, UPR, and Viral Infections
The status of cellular ER stress may play a crucial role in determining whether stressed cells will survive SARS-CoV-2 infection or undergo programmed cell death. The transition from pre-existing acute stress to chronic stress following SARS-CoV-2 infection is a complex process that can vary depending on specific circumstances such as the nature of stressors, duration of stress, and the capacity of infected host cells to cope with the stress. Activation of UPR during a pre-existing ER stress may represent an early host defense mechanism to restore normal cell function and ER homeostasis by promoting the correct folding and processing of proteins and lipids, ultimately leading to reduced SARS-CoV-2 entry into host cells. If the stress is not resolved quickly and remains persistent and prolonged due to UPR hyperactivity and inadequate cellular repair mechanisms, stressed cells become more prone to virus infection, which can cause an aggravated pathological chronic ER stress-associated cellular dysfunction, ultimately affecting the clustering and localization of viral entry receptors on the cell surface, impairing SARS-Co-V-2 entry into host cells.
Understanding ER Stress, UPR, and Viral Infections
ER stress occurs when the protein folding capacity of the ER is overwhelmed, leading to the accumulation of misfolded proteins. In response, cells activate the UPR, a complex signaling pathway aimed at restoring ER homeostasis. Viral infections, including those caused by SARS-CoV-2, can induce ER stress as part of their replication strategies. This activation of the UPR has dual effects, aiding viral replication while also triggering cellular defense mechanisms.
Experimental Approach and Findings
The study team conducted a series of experiments to investigate the impact of acute pre-existing ER stress on SARS-CoV-2 infection. They utilized Huh-7 cells, a commonly used cell line in virology research, and treated them with THA and TUN before exposing them to SARS-CoV-2 pseudo-typed particles (SARS-CoV-2pp).
The results were intriguing. Both THA and TUN effectively inhibited the entry of SARS-CoV-2pp into host cells without causing any cytotoxic effects. This inhibition was dose-dependent, highlighting the specificity of these ER stress-inducing compounds in modulating viral entry. Importa
ntly, this inhibition was independent of ACE2 expression, a critical receptor for SARS-CoV-2 entry.
The key findings of the study included:
-Inhibition of SARS-CoV-2 Entry: Both THA and TUN significantly inhibited SARS-CoV-2pp entry into Huh-7 cells in a dose-dependent manner, highlighting the potential of ER stress inducers as antiviral agents.
-Differential Modulation of UPR: Pre-existing ER stress altered UPR gene markers and suppressed activation of UPR pathways in transduced cells. THA and TUN exhibited distinct effects on UPR signaling, suggesting a nuanced response to ER stressors.
-Impact on Metabolic Signaling: THA enhanced insulin-mediated glucose uptake and phosphorylation of AKT, indicating its role in modulating host metabolic responses. TUN showed differential effects on insulin signaling pathways, providing insights into the complex interplay between ER stress and cellular metabolism.
-Influence on Lipid Metabolism and Apoptosis: THA disrupted lipid droplet formation and lipoprotein secretion, showcasing its broader impact on cellular processes. Moreover, pre-existing ER stress influenced apoptotic pathways, with THA showing distinct effects on key regulators of apoptosis compared to TUN.
Impact on UPR and Host Signaling Pathways
Further analysis revealed that pre-existing ER stress altered the expression of UPR gene markers in transduced cells. The activation of UPR pathways, such as those involving Inositol-requiring enzyme-1 (IRE1), activating transcription factor 6 (ATF6), and protein kinase R-like ER protein kinase (PERK), was suppressed. This modulation of UPR signaling hinted at the intricate interplay between acute ER stress and viral infection.
Moreover, THA and TUN exhibited differential effects on insulin signaling pathways, glucose uptake, lipid metabolism, and apoptosis. THA, in particular, enhanced insulin-mediated glucose uptake and phosphorylation of AKT, suggesting its role in modulating host metabolic responses. These findings underscored the complex and multifaceted nature of host-virus interactions during SARS-CoV-2 infection.
Insights into Lipid Metabolism and Apoptosis
In addition to metabolic signaling, the researchers also investigated the impact of acute pre-existing ER stress on lipid metabolism and apoptotic pathways. THA disrupted lipid droplet formation and lipoprotein secretion, indicating its broader influence on cellular processes. Moreover, pre-existing ER stress influenced apoptotic pathways, with THA showing distinct effects on key regulators of apoptosis compared to TUN.
Discussion and Future Perspectives
The findings from this study provide valuable insights into the role of acute pre-existing ER stress in modulating SARS-CoV-2 infection. The differential effects of THA and TUN on UPR pathways, metabolic signaling, and apoptosis open avenues for targeted therapeutic strategies. Understanding how UPR activation during ER stress affects viral entry and host cell responses is crucial for developing effective treatments against COVID-19.
Future research directions may include investigating the specific molecular mechanisms underlying the observed effects of THA and TUN on UPR pathways and metabolic signaling. Additionally, exploring the impact of acute ER stress on other cell types relevant to SARS-CoV-2 infection could provide a more comprehensive understanding of host-virus interactions.
Conclusion
In conclusion, the study highlights the intricate interplay between acute pre-existing ER stress and SARS-CoV-2 infection. The differential effects of THA and TUN on host responses underscore the complexity of host-virus interactions during viral infections. Further research in this area is warranted to elucidate the underlying mechanisms and develop targeted therapeutic interventions for COVID-19.
The study findings of the research conducted by scientist from King Abdullah International Medical Research Center (KAIMRC)-Saudi Arabia, King Saud bin Abdulaziz University for Health Sciences (KSAU-HS)-Saudi Arabia and King Abdulaziz Hospital-Saudi Arabia were published in the peer reviewed journal: Cells.
https://www.mdpi.com/2073-4409/13/9/769
For the latest
COVID-19 News, keep on logging to Thailand Medical News
