The F17 protein of all Poxviruses including Mpox counteracts mitochondrially orchestrated antiviral responses
Nikhil Prasad Fact checked by:Thailand Medical News Team Sep 02, 2024 7 months, 3 weeks, 3 days, 13 hours, 53 minutes ago
Medical News: A past study conducted at Northwestern University's Feinberg School of Medicine, uncovered significant insights into how poxviruses, including the virus responsible for Mpox (previously known as monkeypox), evade the human body's antiviral defenses. This
Medical News report will delve into the crucial role played by the F17 protein in these viruses, with a particular focus on how it counteracts antiviral responses orchestrated by mitochondria, the energy-producing structures within cells.
 The F17 protein of all Poxviruses including Mpox counteracts mitochondrially orchestrated antiviral responses
Understanding the Poxvirus and Its Defense Mechanisms
The F17 protein of all Poxviruses including Mpox counteracts mitochondrially orchestrated antiviral responses
Understanding the Poxvirus and Its Defense Mechanisms
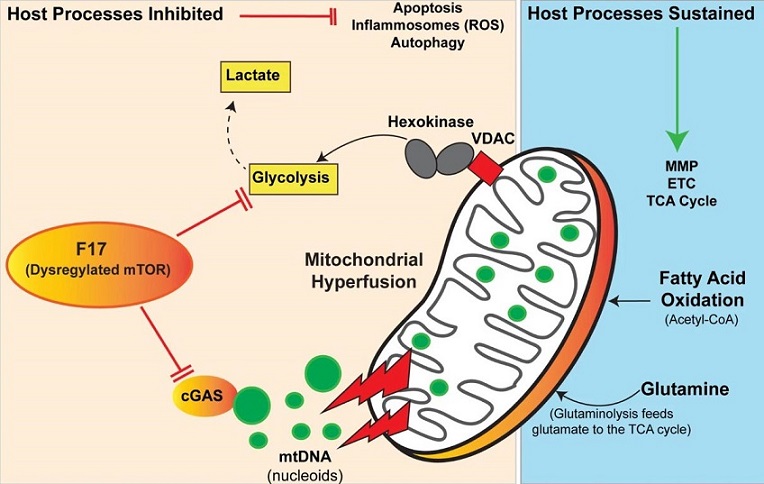
Poxviruses are unique among DNA viruses because they replicate entirely within the cytoplasm of the host cell, away from the nucleus where the cell’s DNA is located. This unusual replication process makes them vulnerable to the host cell's immune defenses, particularly the detection of foreign DNA in the cytoplasm by immune sensors like cGAMP synthase (cGAS). To successfully replicate, poxviruses have developed an array of strategies to counteract these defenses. One of the most intriguing findings from this research is the role of the F17 protein in evading these mitochondrial-based antiviral responses.
The researchers, led by Nathan Meade, Helen K. Toreev, Ram P. Chakrabarty, and their colleagues, have discovered that after the initial stages of infection, the poxvirus lifecycle is closely monitored by mitochondria. These organelles, known for their role in energy production, also coordinate various aspects of the immune response. Typically, viral infections cause significant damage to mitochondria, but poxviruses have evolved to sustain mitochondrial function while reducing the production of reactive oxygen species (ROS), which are known to drive inflammation.
The Role of Mitochondria in Antiviral Responses
Mitochondria are not only the powerhouses of the cell but also critical players in the immune response to infections. When a virus infects a cell, mitochondria can release mitochondrial DNA (mtDNA) into the cytoplasm. This mtDNA acts as a danger signal, alerting the immune system and triggering a cascade of antiviral responses, including the production of interferon-stimulated genes (ISGs). These ISGs are essential for mounting an effective defense against viral infections.
Interestingly, the study revealed that during poxvirus infection, mitochondria undergo a process called hyperfusion. This process leads to the release of mtDNA, which in turn primes the immune system to fight the infection. However, the poxvirus has a countermeasure - the F17 protein. This protein specifically localizes to the mitochondria and disrupts the host cell's ability to mount an effective antiviral response by destabilizing cGAS and inhibiting glycolysis, a metabolic pathway essential for the production of ISGs.
F17 Protein: The Viral Weapon Against Mitochondrial Defenses
The F17 protein is a structural comp
onent of the poxvirus core and is critical for the formation of new viral particles. However, its role extends beyond mere structural support. The researchers found that F17 directly interacts with the mammalian/mechanistic Target of Rapamycin (mTOR) pathway, a key regulator of cellular metabolism and immune responses. By binding to and dysregulating mTOR, F17 effectively blocks the host cell's ability to produce ISGs in response to the release of mtDNA.
This study findings highlight the critical discovery that F17 uses a dual strategy to evade the host's immune response. First, it destabilizes cGAS, preventing the initial detection of viral DNA. Second, it inhibits the increase in glycolysis that is necessary for sustaining the production of ISGs. These findings shed light on the sophisticated mechanisms poxviruses use to ensure their survival and replication within host cells.
The Broader Implications of F17's Role in Viral Evasion
The discovery of F17's role in counteracting mitochondrial antiviral responses has significant implications for our understanding of poxvirus infections, including Mpox. By targeting the mTOR pathway, F17 not only disrupts the cell's immune response but also alters the metabolic state of the cell, making it more favorable for viral replication. This multifaceted approach to immune evasion highlights the complexity of the interactions between viruses and their host cells.
Moreover, the study's findings suggest that F17 is conserved across various poxviruses, indicating its importance in the viral lifecycle. This conservation makes F17 a potential target for therapeutic interventions aimed at disrupting the virus's ability to evade the immune system. By inhibiting F17, it may be possible to enhance the body's natural antiviral responses, leading to more effective treatments for poxvirus infections.
Conclusions and Future Directions
The study conducted by researchers at Northwestern University provides critical insights into the role of the F17 protein in poxvirus infections. By counteracting mitochondrially orchestrated antiviral responses, F17 plays a vital role in the virus's ability to replicate and evade the host's immune defenses. The findings of this study not only advance our understanding of poxvirus biology but also open up new avenues for therapeutic research.
As the scientific community continues to explore the intricate interactions between viruses and host cells, the knowledge gained from this study could lead to the development of novel antiviral strategies. Targeting the F17 protein and its interactions with the mTOR pathway may offer a new approach to combating poxvirus infections, including emerging threats like Mpox.
The study findings were published in the peer-reviewed journal Nature Communications.
https://link.springer.com/article/10.1038/s41467-023-43635-y
For the latest Mpox News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/study-discovers-that-mpox-virus-protein-h3l-induces-transcriptional-perturbations-and-cellular-injuries
https://www.thailandmedical.news/news/the-structural-and-functional-insights-of-the-e5-helicase-protein-of-the-mpox-virus
https://www.thailandmedical.news/news/the-mpox-virus-s-f3-protein-triggers-immune-responses-and-apoptosis
https://www.thailandmedical.news/news/unveiling-the-role-of-monkeypox-mpox-virus-a23-protein-in-human-cells
