The Role Of Cytotoxic Lymphocyte-Monocyte Complexes In COVID-19 Immune Responses
Nikhil Prasad Fact checked by:Thailand Medical News Team Apr 28, 2024 1 year, 7 months, 2 weeks, 2 days, 14 hours, 55 minutes ago
COVID-19 News: The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has presented a complex array of clinical manifestations, many of which stem from altered immune responses, both locally and systemically. While immune cell crosstalk is a fundamental aspect of immune response, specific interactions occurring in the context of COVID-19 have not been thoroughly characterized. In this
COVID-19 News report, we delve into a groundbreaking study conducted by researchers at Shanghai Jiao Tong University School of Medicine, China. Through the use of advanced techniques such as single-cell RNA sequencing and imaging flow cytometry analysis, the study sheds light on a previously unrecognized phenomenon - the formation of cytotoxic lymphocyte-monocyte complexes (CLM) in the peripheral blood, revealing their dynamic role in the systemic immune response to SARS-CoV-2 infection.
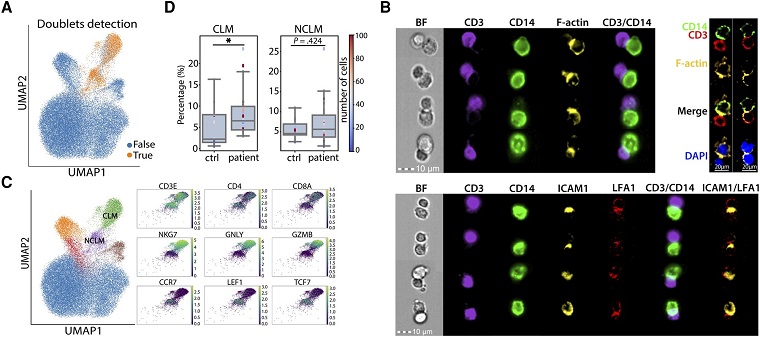 Role Of Cytotoxic Lymphocyte-Monocyte Complexes In COVID-19 Immune Responses. Monocyte doublets detected in myeloid cell populations were associated with severe acute respiratory syndrome coronavirus 2 infection. A, Uniform manifold approximation and projection (UMAP) showing the cell doublets predicted by DoubletFinder. B, Microscope images assessing the content distribution of F-actin (top) and ICAM1/LFA1 (bottom) in CD3+CD14+ compartments. C, UMAPs showing the annotation of CD3+CD14+ clusters (left) and the expression of essential signature genes (right). D, Box plots showing the percentage of CLM complex (left) and NCLM complex (right) in healthy individuals (ctrl) and COVID-19 patients (patient). *P < .05 (1-tailed Wilcoxon rank-sum test). Each dot represents an individual donor. Color scale of dots represents the number of cell complexes. Abbreviations: CLM complex, cytotoxic lymphocyte-monocyte complex; NCLM complex, noncytotoxic lymphocyte-monocyte complex; ctrl, control.
The Importance of Immune Cell Interactions
Role Of Cytotoxic Lymphocyte-Monocyte Complexes In COVID-19 Immune Responses. Monocyte doublets detected in myeloid cell populations were associated with severe acute respiratory syndrome coronavirus 2 infection. A, Uniform manifold approximation and projection (UMAP) showing the cell doublets predicted by DoubletFinder. B, Microscope images assessing the content distribution of F-actin (top) and ICAM1/LFA1 (bottom) in CD3+CD14+ compartments. C, UMAPs showing the annotation of CD3+CD14+ clusters (left) and the expression of essential signature genes (right). D, Box plots showing the percentage of CLM complex (left) and NCLM complex (right) in healthy individuals (ctrl) and COVID-19 patients (patient). *P < .05 (1-tailed Wilcoxon rank-sum test). Each dot represents an individual donor. Color scale of dots represents the number of cell complexes. Abbreviations: CLM complex, cytotoxic lymphocyte-monocyte complex; NCLM complex, noncytotoxic lymphocyte-monocyte complex; ctrl, control.
The Importance of Immune Cell Interactions
Immune cell interactions are crucial events in orchestrating an effective immune response, involving the secretion of molecular messengers and direct cell-to-cell contact. In the context of COVID-19, these interactions, particularly involving circulating monocytes, play pivotal roles in various stages of disease progression.
Monocytes are known to migrate to sites of injury, recruit cytotoxic T cells and neutrophils, and secrete cytokines, contributing to an augmented inflammatory response and tissue damage. Additionally, as antigen-presenting cells (APCs), monocytes play a vital role in priming adaptive immune cells, ultimately shaping the activation and polarization of T cells specific to the virus.
However, the specific nature of cell interactions in the context of COVID-19 has not been fully elucidated. While single-cell RNA sequencing has been a powerful tool to infer cellular interactions based on ligand-receptor pairs, it often overlooks the presence of cell complexes. The study aims to address this limitation by exploring the formation and functional
significance of CLMs, shedding light on the intricate interplay between monocytes and cytotoxic lymphocytes during SARS-CoV-2 infection.
Unveiling Cytotoxic Lymphocyte-Monocyte Complexes
The research involved the analysis of single-cell RNA sequencing data from peripheral blood mononuclear cells (PBMC) of COVID-19 patients and healthy donors. The data revealed the existence of a heterogeneous group of cell complexes, termed cytotoxic lymphocyte-monocyte complexes (CLM), formed by the adherence of CD14+ monocytes to various cytotoxic lymphocytes, including SARS-CoV-2-specific CD8+ T cells, γδT, and NKT cells.
Notably, the abundance of granulysin-expressing CD8 effector T cells was found to be a predominant subpopulation specific to SARS-CoV-2 infection. These cells underwent significant clonal expansion, highlighting their crucial role in the adaptive immune response to the virus.
Functional Characteristics of CLM in Disease Progression
In the disease progression stage, CLM complexes exhibited enhanced inflammasome assembly, viral genome replication, and pyroptosis-induced cell death. The analysis suggested that monocytes within CLMs possibly underwent SARS-CoV-2 infection, leading to perturbations in cellular homeostasis, cytosolic trafficking, and glucose metabolism. The upregulation of genes associated with viral infection, such as ADAM17, RAB7, and HIF1A, provided further insights into
the potential mechanisms driving the enhanced inflammatory response.
The involvement of CLMs in the progression stage raised questions about the dynamics of these complexes during the recovery phase. Surprisingly, CLMs persisted in convalescent samples, indicating their involvement in a unique immune response throughout the course of infection.
Adaptive Changes in CLM Complexes during Convalescence
In the convalescent stage, CLMs displayed an altered composition, with CD14+ monocytes adhering to various cytotoxic lymphocytes, including CD8 Teff-GNLY, NKT, γδT, and CD4 Teff-GNLY. Intriguingly, CLMs in recovery showed a decrease in the expression of CD14 and an increase in the expression of markers associated with dendritic cells and macrophages, suggesting a differentiation of monocytes towards these immune cell types.
Furthermore, CLMs in convalescence exhibited an enhanced MHC-II complex assembly, indicating a shift towards antigen presentation. The downregulation of MHC-I molecules and costimulatory receptors suggested a reduced priming of cytotoxic lymphocytes by monocytes in the recovery stage. Interestingly, the accumulation of small UMI cells and upregulation of apoptosis-related genes in CLMs of convalescence hinted at a negative regulation of antigen-presenting cells by cytotoxic lymphocytes.
Implications and Future Perspectives
This comprehensive study provides novel insights into the dynamics of immune cell interactions during SARS-CoV-2 infection. The discovery of CLMs and their distinct roles in disease progression and recovery expands our understanding of the intricate interplay between monocytes and cytotoxic lymphocytes.
The persistence of CLMs in convalescent samples challenges conventional notions of immune response resolution, suggesting a potential role for cytotoxic lymphocytes in eliminating excessive antigen-presenting cells to prevent sustained immune activation. However, the mechanisms underlying the inefficient killing capability of cytotoxic lymphocytes in CLMs during recovery warrant further investigation.
Understanding the molecular pathways and signaling involved in CLM formation and function could offer valuable targets for therapeutic interventions in COVID-19. Additionally, exploring the potential implications of CLMs in local tissues versus the bloodstream may uncover nuances in the spatial and temporal aspects of immune regulation during SARS-CoV-2 infection.
In conclusion, the study provides a foundation for future research aimed at unraveling the complexities of immune responses to viral infections, offering potential avenues for therapeutic strategies and advancing our knowledge of the immunopathogenesis of COVID-19.
The study findings were published in the peer reviewed Journal of Infectious Diseases.
https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiae048/7596101
For the latest
COVID-19 News, keep on logging to Thailand Medical News
