Nikhil Prasad Fact checked by:Thailand Medical News Team Apr 04, 2024 1 year, 8 months, 2 weeks, 6 days, 6 hours, 56 minutes ago
COVID-19 News: The outbreak of SARS-CoV-2, the virus responsible for COVID-19, has led to a global health crisis with diverse clinical manifestations. Apart from the well-documented respiratory symptoms, SARS-CoV-2 has been associated with a spectrum of neurological disorders, ranging from headaches and dizziness to more severe conditions like cerebrovascular diseases and encephalopathy.
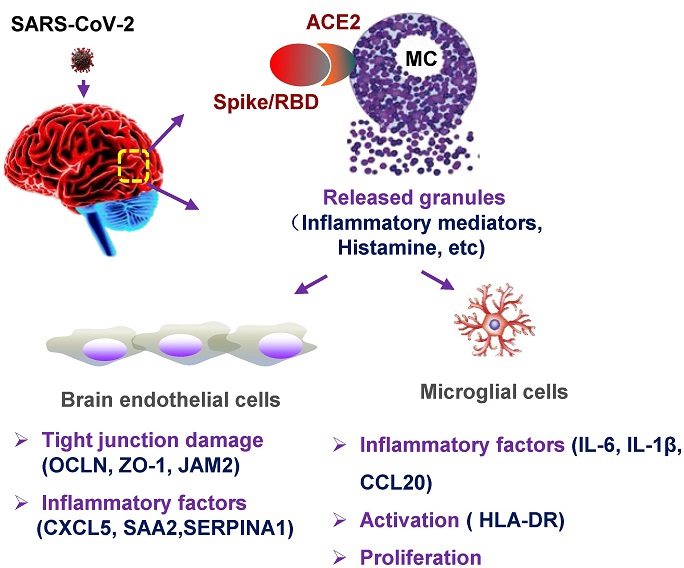 Role Of Mast Cells In SARS-CoV-2 Induced Neuroinflammation
Graphical abstract. MC degranulation causes inflammation in brain microvascular endothelial cells and microglia. The treatment with SARS-CoV-2 spike protein triggers MC degranulation, and the degranulated component such as histamine induces the inflammatory factors CXCL5, SAA2, SERPINA1 in human brain microvascular endothelial cells, and reduced the tight junction proteins of OCLN, ZO-1, and JAM2; MC degranulation also increases the expression of inflammatory factors IL-6, IL-1β, and CCL20 in microglial cells, and induces the activation and proliferation of microglia.
Role Of Mast Cells In SARS-CoV-2 Induced Neuroinflammation
Graphical abstract. MC degranulation causes inflammation in brain microvascular endothelial cells and microglia. The treatment with SARS-CoV-2 spike protein triggers MC degranulation, and the degranulated component such as histamine induces the inflammatory factors CXCL5, SAA2, SERPINA1 in human brain microvascular endothelial cells, and reduced the tight junction proteins of OCLN, ZO-1, and JAM2; MC degranulation also increases the expression of inflammatory factors IL-6, IL-1β, and CCL20 in microglial cells, and induces the activation and proliferation of microglia.
Furthermore, the post-acute sequelae of SARS-CoV-2, known as Long-COVID syndrome, has raised concerns due to persistent neurological and cognitive deficits observed in convalescent patients.
Brain autopsies of COVID-19 patients have revealed significant pathological findings, including hemorrhage, vascular congestion, and intravascular platelet aggregates, suggesting a direct impact of the virus on the central nervous system (CNS). Neuroinflammation, characterized by an excessive immune response in the brain, has emerged as a critical factor contributing to the neurological complications of COVID-19. This
COVID-19 News report delves into the cellular mechanisms underlying SARS-CoV-2-induced neuroinflammation, particularly focusing on the role of mast cells (MCs) in this process based on study findings by a new research by scientists from Chinese Academy of Sciences, Guangzhou-China, The First Affiliated Hospital of Guangzhou Medical University-Guangdong, China and the University of Chinese Academy of Sciences, Beijing-China.
The Role of Mast Cells in Neuroinflammation
Mast cells, traditionally recognized for their involvement in allergic reactions, are increasingly acknowledged as key regulators of immune responses in various tissues, including the brain. Positioned near the neurovascular region within the brain, mast cells play a pivotal role in sensing pathogens and initiating immune reactions. In the context of SARS-CoV-2 infection, recent studies have highlighted the significant accumulation and activation of mast cells, particularly in the lungs of infected individuals.
Research has demonstrated that SARS-CoV-2-induced mast cell activation in the lungs leads to the release of inflammatory mediators, contributing to lung inflammation and damage. This activation and subsequent release of cytokines and other molecules have been proposed to extend beyond the lungs, affecting distant organs such as the brain. The proximity of
mast cells to the blood-brain barrier (BBB) makes them potential instigators of neuroinflammation in the CNS following viral infections.
SARS-CoV-2 and Mast Cell Activation in the Brain
Examining the effects of SARS-CoV-2 infection on mast cells in the CNS, studies have shown a marked accumulation of mast cells around the cerebrovascular region in infected mice. This observation suggests a direct influence of the virus on mast cell dynamics within the brain, potentially triggering neuroinflammatory processes.
Further investigations into the mechanisms of mast cell activation in response to SARS-CoV-2 revealed that viral components, particularly the spike protein and its receptor-binding domain (RBD), can stimulate mast cells to undergo degranulation. This process results in the release of inflammatory factors that can impact neighboring brain cells, including microglia and brain microvascular endothelial cells.
Inflammatory Cascade and Microglial Activation
The released mediators from degranulated mast cells have been shown to induce the expression of inflammatory cytokines in human brain microvascular endothelial cells and microglia. This inflammatory cascade, characterized by increased levels of IL-6, IL-8, TNF-α, and other factors, contributes to neuroinflammation and the activation of microglial cells.
Microglial activation, a common pathological feature observed in COVID-19-related brain autopsies, is further exacerbated by mast cell degranulation. The interaction between mast cell-derived factors and microglia leads to cellular proliferation and the release of additional neurotoxic mediators, perpetuating the cycle of neuroinflammation and neuronal damage.
Disruption of Blood-Brain Barrier Integrity
A critical aspect of SARS-CoV-2-induced neuroinflammation is the disruption of the blood-brain barrier (BBB). Mast cell degranulation has been shown to impair the integrity of tight junction proteins in brain microvascular endothelial cells, compromising the BBB's protective function. This disruption allows for the infiltration of inflammatory cells into the CNS, contributing to brain edema and hemorrhage observed in severe cases of COVID-19.
Transcriptome Analysis and Cellular Signaling Alterations
Transcriptome analysis of brain microvascular endothelial cells exposed to mast cell degranulation products revealed significant alterations in cellular signaling pathways. Upregulation of genes associated with inflammation response and downregulation of genes involved in cell adhesion and junction organization were observed, providing insights into the molecular mechanisms underlying SARS-CoV-2-induced neuroinflammation.
Implications and Future Directions
The findings from these studies shed light on the intricate interplay between SARS-CoV-2 infection, mast cell activation, and neuroinflammation. Understanding these cellular mechanisms is crucial for developing targeted therapeutic interventions to mitigate neurological complications associated with COVID-19.
Future research directions may include investigating the efficacy of mast cell stabilizers or anti-inflammatory agents in attenuating neuroinflammation and preserving BBB integrity in COVID-19 patients. Additionally, exploring the long-term neurological outcomes and potential neurodegenerative effects of SARS-CoV-2-induced neuroinflammation is essential for comprehensive patient management and care.
In conclusion, the intricate network of cellular interactions involving mast cells, brain microvascular endothelial cells, and microglia plays a significant role in mediating neuroinflammation in the context of SARS-CoV-2 infection. Unraveling these mechanisms provides valuable insights into the pathophysiology of COVID-19-related neurological complications and opens avenues for targeted therapeutic strategies aimed at preserving brain health in affected individuals.
The study findings were published in the peer reviewed journal: Frontiers in Cellular and Infection Microbiology.
https://www.frontiersin.org/articles/10.3389/fcimb.2024.1358873/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
