Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 03, 2024 5 months, 1 week, 3 days, 15 hours, 59 minutes ago
Medical News: Researchers from Universidad de La Frontera (Chile), Universidade de São Paulo (Brazil), and Universidad Santo Tomás (Chile) have conducted a promising study on the potential neuroprotective effects of thyroid hormone therapy in addressing Alzheimer’s-like symptoms. Alzheimer’s disease, the most prevalent form of dementia, primarily affects cognitive functions, leading to memory loss and significant neuroinflammation. This recent study suggests that thyroid hormone supplementation, specifically triiodothyronine (T3), could help reduce these neurodegenerative effects.
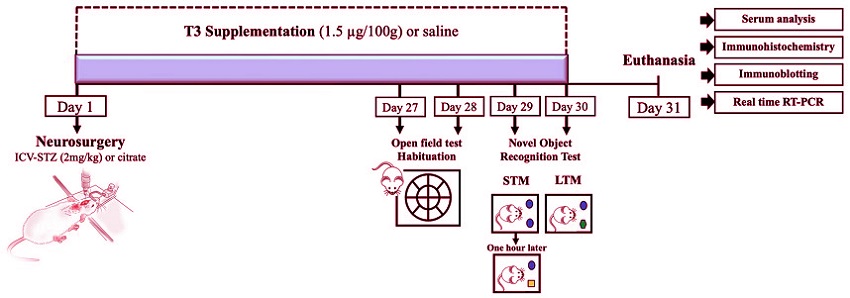 Graphical representation of the experimental design. The establishment of a streptozotocin (STZ)-induced sporadic Alzheimer’s disease (sAD) animal model, T3 supplementation, and experimental procedures are shown. STM - short-term memory; LTM - long-term memory.
Thyroid Hormone and Alzheimer’s Disease
Graphical representation of the experimental design. The establishment of a streptozotocin (STZ)-induced sporadic Alzheimer’s disease (sAD) animal model, T3 supplementation, and experimental procedures are shown. STM - short-term memory; LTM - long-term memory.
Thyroid Hormone and Alzheimer’s Disease
Thyroid hormones, particularly T3, are essential for brain development and cognitive functions. Imbalances in thyroid hormones have been linked to neurological disorders, including Alzheimer’s. This
Medical News report explores how T3 supplementation might offer neuroprotection by enhancing insulin signaling in the brain and reducing neuroinflammation, particularly in the hippocampus - a brain region critical to memory and cognitive function.
The study used a rat model to simulate sporadic Alzheimer’s disease (sAD) by injecting the animals with streptozotocin (STZ). The rats were then treated with T3 for 30 days to observe changes in memory, insulin signaling pathways, and markers of neuroinflammation and apoptosis.
Methodology and Experiment Design
The study involved administering STZ to male Wistar rats, a common model for replicating Alzheimer’s symptoms. The rats were split into four groups: control with no STZ or T3, T3 only, STZ only, and a final group receiving both STZ and T3 treatment. Over a 30-day period, researchers measured several key biological markers, such as memory performance, levels of inflammatory cytokines, insulin signaling indicators, and proteins involved in cell survival and apoptosis.
Key Findings
Improved Memory and Cognitive Function
The novel object recognition test showed that T3-treated rats had improved memory, indicating cognitive function restoration. This test, which assesses recognition memory, revealed that T3 supplementation helped reverse the cognitive impairments observed in rats given STZ alone.
Enhanced Insulin Signaling
Insulin resistance, often observed in Alzheimer’s, disrupts the brain’s energy usage and neuronal health. In this study, T3 enhanced insulin signaling in the brain by increasing phosphorylation of Akt - a protein kinase vital for cell survival and neuroplasticity. The STZ-only group showed impaired insulin signaling, but T3-treated rats displayed improved Akt activation, suggesting that T3 may help n
ormalize brain insulin pathways.
Reduced Neuroinflammation
Elevated levels of inflammatory cytokines are linked to Alzheimer’s-related neurodegeneration. The study found that T3 treatment led to lower levels of key inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukins (IL-6 and IL-1β), in the hippocampus. Immunohistochemical analysis showed reduced expression of these cytokines in the T3-treated group, indicating that T3 supplementation may counteract neuroinflammation in Alzheimer’s.
Increased Anti-apoptotic Markers
Apoptosis, or programmed cell death, is accelerated in Alzheimer’s due to the activation of certain pro-apoptotic proteins. T3-treated rats showed increased expression of the anti-apoptotic protein Bcl-2 and decreased levels of the pro-apoptotic protein BAX. This adjustment in protein expression suggests that T3 could help prevent cell death in the brain, potentially preserving neuronal health and cognitive function in Alzheimer’s disease.
Boosted BDNF Gene Expression
Brain-derived neurotrophic factor (BDNF) is critical for neuronal survival and plasticity. The STZ-only group had reduced BDNF levels in the hippocampus, a change associated with cognitive decline. However, T3 treatment increased BDNF expression, potentially supporting brain health and mitigating Alzheimer’s-related cognitive impairment.
Enhanced Expression of MCT8 Transporter
The MCT8 transporter is essential for transporting thyroid hormones across the blood-brain barrier. T3-treated rats showed an increase in MCT8 expression in the hippocampus, indicating improved transport of thyroid hormones into the brain. This effect could be beneficial in Alzheimer’s treatment, as it allows more thyroid hormone to reach brain regions where it can exert neuroprotective effects.
Conclusions
The findings of this study are highly promising, highlighting T3’s potential as a neuroprotective therapy for Alzheimer’s disease. By improving cognitive function, reducing inflammation, and enhancing insulin signaling, T3 supplementation could offer a multifaceted approach to managing Alzheimer’s symptoms. However, further research is essential to understand how T3 interacts with other brain pathways and its long-term safety in humans. For individuals at risk of Alzheimer’s, particularly those with thyroid imbalances, T3 supplementation might one day become a viable treatment to slow disease progression or alleviate symptoms.
The study findings were published in the peer-reviewed journal: Cells.
https://www.mdpi.com/2073-4409/13/21/1793
For the latest on Alzheimer, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/scientists-from-singapore-discover-plasma-biomarkers-that-identify-blood-brain-barrier-dysregulation-in-alzheimer-s-disease
https://www.thailandmedical.news/news/breaking-news-study-reveals-potential-new-form-of-brain-neurodegeneration-linked-to-covid-19
