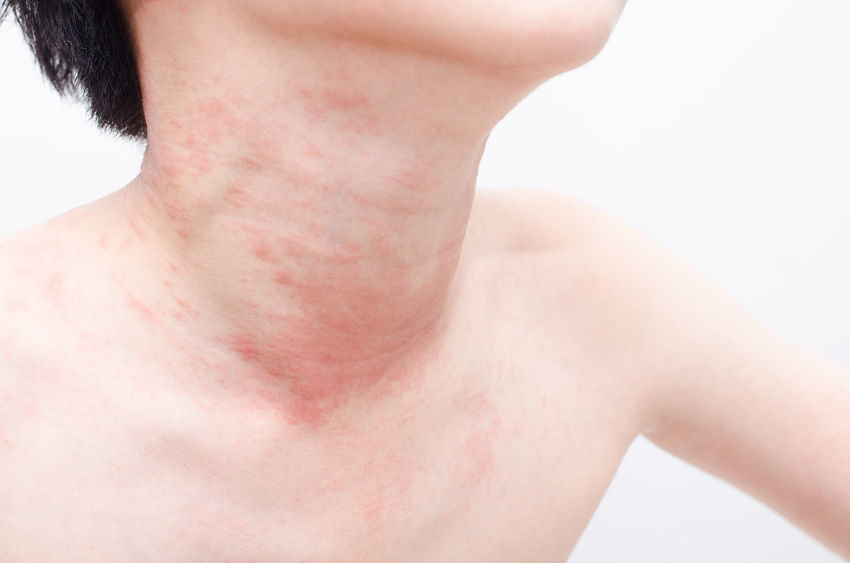
TNF Inhibitors Use In Children With Inflammatory Diseases Linked To Higher Incidence Of Psoriasis
Source: Thailand Medical News Nov 08, 2019 5 years, 5 months, 2 weeks, 4 days, 20 hours, 37 minutes ago
Researchers from the Children’s Hospital of Philadelphia have discovered that children with either
inflammatory bowel disease, chronic noninfectious osteomyelitis or juvenile idiopathic arthritis developed
psoriasis at an increased rate compared with the general public, with the highest rates seen among those treated with
TNF inhibitors.
Dr Lisa H. Buckley of the Children’s Hospital of Philadelphia told
Thailand Medical News in a phone interview, “To date, the incidence rate (IR) and risk factors of
psoriasis in children with IBD, JIA or CNO who are exposed to
TNF inhibitors are unknown. Additionally, there is a well-established association between these
inflammatory conditions and psoriasis development. Yet, as TNF inhibitors can both treat and trigger
psoriasis, it is not clear how
TNF inhibitor exposure affects this relationship. To date, the pediatric literature primarily consists of case reports and series without the use of a comparison group.”
In order to calculate the incidence rate of psoriasis among children with IBD, JIA and chronic noninfectious osteomyelitis compared with the general population, as well as among patients treated with
TNF inhibitors compared with those who were not, Buckley and colleagues conducted a retrospective cohort study of electronic health data at the Children’s Hospital of Philadelphia. The researchers analyzed data on 4,111 children with either of the three diseases who were evaluated at the center between Jan. 1, 2008, and Oct. 1, 2018.
Children were determined to have been exposed to
TNF inhibitors if they had received a prescription for adalimumab (Humira, AbbVie), etanercept (Enbrel, Amgen), infliximab (Remicade, Janssen), certolizumab (Cimzia, UCB) or golimumab (Simponi, Janssen). The primary outcome was incident
psoriasis. The researchers estimated incidence rates and standardized incidence ratios, and used Cox proportional hazards models to determine the links between
psoriasis with
TNF inhibitor exposure and other risk factors.
Also in the included patients, were 39% that had been treated with
TNF inhibitors. Total follow-up was 6,604 person-years.
The findings showed that there were 58 cases of psoriasis in patients treated with
TNF inhibitors, resulting in an incidence rate of 12.3 per 1,000 personyears, and 25 cases of psoriasis among those without exposure to the drug, making for an incidence rate of 3.8 per 1,000 personyears cases. The overall standardized incidence ratio was 18 (95% CI, 15-22); 30 (95% CI 23, 39) for patients treated with
TNF inhibitors, and 9.3 (95% CI, 6.3-14) for those without
TNF inhibitor exposure. Additionally, the hazard ratio for
psoriasis among juvenile patients treated with
TNF inhibitors, compared with those who were not, was 3.84 (95% CI, 2.28-6.47).
Dr Lisa H. Buckley added, “This study demonstrates that children with IBD,&
;nbsp;JIA and CNO had an increased rate of
psoriasis compared to the general pediatric population, with the highest rate in those with
TNF inhibitor exposure. It is the first to estimate the IR of
TNF inhibitor-associated psoriasis and to formally evaluate the strength of association between
TNF inhibitor exposure and
psoriasis.”
The researchers commented “There is a paucity of data regarding this adverse cutaneous event in children, therefore this study contributes significantly to the current literature. Despite limitations, the results are highly significant and indicate the need for additional studies to include long-term data from large observational registries.”
Reference: Psoriasis risk spikes with anti-TNF use in children with inflammatory diseases
Buckley LH, et al. Arthritis Care Res. 2019;doi:10.1002/acr.24100.
