To evade the immune system and antibodies, SARS-CoV-2 RBD switches to the down conformation
Nikhil Prasad Fact checked by:Thailand Medical News Team Jul 14, 2024 8 months, 4 weeks, 2 days, 12 hours, 20 minutes ago
COVID-19 News: The ongoing battle between the human immune system and the SARS-CoV-2 virus has revealed a complex game of hide and seek. New research has unveiled a fascinating tactic employed by the virus to evade immune detection, involving a conformational switch of its spike protein. This
COVID-19 News report delves into the intricate details of this discovery, explaining how the virus outmaneuvers our immune defenses.
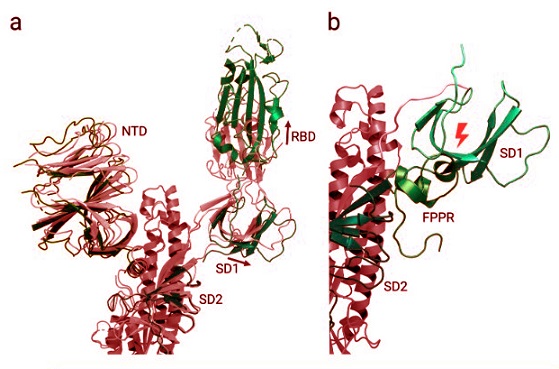 Domain shifts between the up and down conformations of the spike protein receptor binding domain. Abbreviations: NTD: N-terminal domain, RBD: receptor binding domain, SD1: sub-domain 1, SD2: sub-domain 2; FPPR: fusion peptide proximal region. (a) Superimposed spike protein structures with receptor binding domains in up (PDB 6VSB; coloured by domain) and down (PDB6VXX; coloured red) conformations. The fusion peptide proximal region is not resolved in either structure. With the movement of the receptor binding domain into the ‘up’ conformation. (b) Superimposed spike structures with receptor binding domains in up (PDB 6VSB; colours as in a) and down (PDB 6XR8; green) conformations; for 6XR8 only the fusion peptide proximal region is shown. In contrast to (a), the fusion peptide proximal region is resolved in6XR8. When the receptor binding domain switches into ‘up’ conformation, the SD1 domain rotates out-wards and would then clash with an ordered fusion peptide proximal region, so the FPPR must delocalize in the up conformation.
The Key Players: Spike Protein and RBD
Domain shifts between the up and down conformations of the spike protein receptor binding domain. Abbreviations: NTD: N-terminal domain, RBD: receptor binding domain, SD1: sub-domain 1, SD2: sub-domain 2; FPPR: fusion peptide proximal region. (a) Superimposed spike protein structures with receptor binding domains in up (PDB 6VSB; coloured by domain) and down (PDB6VXX; coloured red) conformations. The fusion peptide proximal region is not resolved in either structure. With the movement of the receptor binding domain into the ‘up’ conformation. (b) Superimposed spike structures with receptor binding domains in up (PDB 6VSB; colours as in a) and down (PDB 6XR8; green) conformations; for 6XR8 only the fusion peptide proximal region is shown. In contrast to (a), the fusion peptide proximal region is resolved in6XR8. When the receptor binding domain switches into ‘up’ conformation, the SD1 domain rotates out-wards and would then clash with an ordered fusion peptide proximal region, so the FPPR must delocalize in the up conformation.
The Key Players: Spike Protein and RBD
The spike protein of SARS-CoV-2, protruding from the virus's surface, plays a critical role in infecting human cells. It binds to the Angiotensin-Converting Enzyme 2 (ACE2) receptors on human cells, initiating the process of viral entry. Due to its crucial role, the spike protein is a primary target for vaccines and therapeutic interventions.
The spike protein is composed of two subunits, S1 and S2. The S1 subunit contains the Receptor Binding Domain (RBD), which is responsible for binding to ACE2 receptors. The RBD can adopt two conformations: "up" and "down." In the "up" position, the RBD is exposed and can interact with ACE2, while in the "down" position, it is less accessible.
Immune Evasion through Conformational Change
Recent research by scientists from the Institut für Nanostruktur und Festkörperphysik at Universität Hamburg-Germany, VU University Amsterdam-Netherlands, the University of Oregon-USA, and others has shown that the SARS-CoV-2 virus can switch the RBD to the "down" conformation to evade immune detection. This tactic makes it harder for antibodies to recognize and neutralize the virus.
Structural Insights into the Spike Protein
The spike protein is a trimer, meaning it is composed of three identical subunits. Each subunit can independently switch between the "up" and "down" conformations. The RBD's abili
ty to hide by adopting the "down" conformation is a key mechanism for immune evasion. When the RBD is in the "down" position, it is less likely to be recognized by antibodies, allowing the virus to escape immune surveillance.
The Role of Glycans
One of the ways the spike protein achieves this camouflage is through glycosylation. Glycans are sugar molecules attached to proteins that can shield the protein from the immune system. The spike protein is heavily glycosylated, with these glycans forming a protective shield over the RBD when it is in the "down" position. This glycan shield is highly flexible and dynamic, making it a formidable barrier against antibody recognition.
Mutations and Variants
The SARS-CoV-2 virus has evolved various mutations that enhance its ability to evade the immune system. Some of these mutations directly affect the spike protein's structure, impacting how easily it can switch between the "up" and "down" conformations. Variants such as Delta and Omicron have shown increased resistance to neutralizing antibodies, partly due to these mutations.
Implications for Vaccine and Therapeutic Development
Understanding the conformational changes of the spike protein has significant implications for vaccine and therapeutic development. Most current vaccines target the spike protein, aiming to elicit an immune response that can neutralize the virus. However, if the virus can switch its RBD to the "down" position, it may evade these responses.
Researchers are now focusing on developing vaccines and therapies that can target the spike protein in both conformations. This includes designing antibodies that can bind to the RBD even when it is in the "down" position, ensuring that the virus cannot escape immune detection.
Future Research Directions
Future research will continue to explore the mechanisms behind the conformational changes of the spike protein and how these can be targeted more effectively. This includes studying the role of different glycan structures and their impact on immune evasion.
Furthermore, understanding how mutations in the spike protein influence its conformational dynamics will be crucial in predicting and countering new variants of the virus. This ongoing research is vital for staying ahead of the virus and ensuring the effectiveness of vaccines and therapies.
Conclusion
The discovery that SARS-CoV-2 can switch its RBD to the "down" conformation to evade the immune system is a significant breakthrough in our understanding of the virus's tactics. By uncovering this mechanism, the researchers have provided crucial insights that will inform future vaccine and therapeutic development.
The study findings were published in the peer-reviewed journal Crystallography Reviews.
https://www.tandfonline.com/doi/full/10.1080/0889311X.2024.2363756
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-covid-19-news-texas-study-finds-that-omicron-variants-utilize-conformational-changes-to-evade-neutralization-by-key-antibodies
https://www.thailandmedical.news/news/breaking-covid-19-news-texas-study-finds-that-omicron-variants-utilize-conformational-changes-to-evade-neutralization-by-key-antibodies
