tRNA Thiouridine Modification Reprogramming Behind Parasite Plasmodium Falciparum Developing Antimalarial Drug Resistance
Nikhil Prasad Fact checked by:Thailand Medical News Team May 18, 2024 1 year, 7 months, 1 week, 22 hours, 20 minutes ago
Medical News: In the perpetual battle between humanity and disease, malaria remains a formidable foe. In 2022 alone, this mosquito-borne illness afflicted around 249 million people worldwide. The spread of malaria, primarily through Anopheles mosquitoes, poses a severe threat to global health, especially given the increasing resistance of the Plasmodium falciparum parasite to antimalarial drugs. Recent groundbreaking research by an interdisciplinary team from the Singapore-MIT Alliance for Research and Technology (SMART), the Massachusetts Institute of Technology (MIT), Columbia University Irving Medical Center, and Nanyang Technological University (NTU), Singapore, has shed new light on the mechanisms behind this resistance. Their findings covered in this
Medical News report, unravel the complex interplay of tRNA thiouridine modification reprogramming and how it equips malaria parasites with the tools to survive antimalarial treatments.
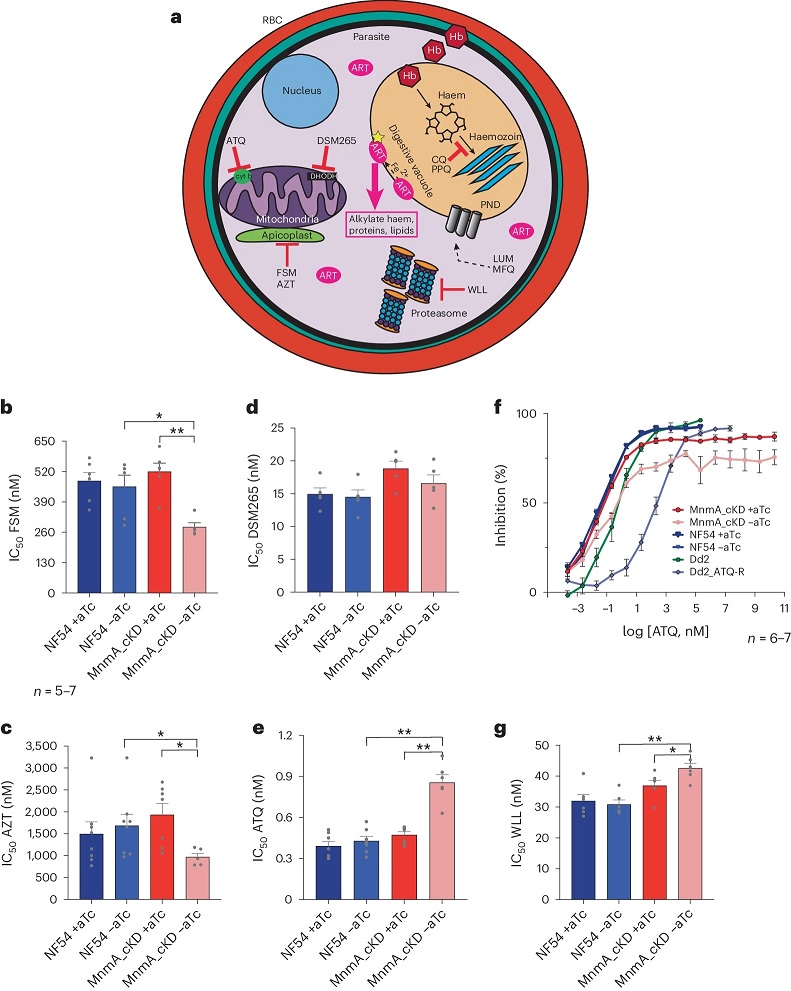 PfMnmA contributes to parasite responses to multiple stressors.
a, A schematic of molecular sites of action for anti-malarials used in this study. Hb, haemoglobin; LUM, lumefantrine; MFQ, mefloquine; PPQ, piperaquine. b–e,g, IC50 data shown as means ± s.e.m. from 72 h dose–response assays of asynchronous NF54 parasites ±aTc, PfMnmA parasites cultured with aTc and PfMnmA parasites cultured without aTc for 96 h before drug pulse (Extended Data Fig. 9a) for FSM (b), AZT (c), DSM265 (d), ATQ (e) and WLL (g). n = 5–7. Statistical significance was determined via two-tailed Mann–Whitney U-tests. *P < 0.05 and **P < 0.01. f, Dose–response curves for ATQ for NF54 parental line with and without aTc, PfMnmA parasites cultured with aTc and PfMnmA parasites cultured without aTc for 96 h before drug pulse, and Dd2 and Dd2_ATQ-R (ATQ resistant, Dd2-CYT1-V259L). The error bars represent s.e.m. n = 6–7 independent biological replicates per parasite line.
The Silent Killer: Malaria's Global Impact
PfMnmA contributes to parasite responses to multiple stressors.
a, A schematic of molecular sites of action for anti-malarials used in this study. Hb, haemoglobin; LUM, lumefantrine; MFQ, mefloquine; PPQ, piperaquine. b–e,g, IC50 data shown as means ± s.e.m. from 72 h dose–response assays of asynchronous NF54 parasites ±aTc, PfMnmA parasites cultured with aTc and PfMnmA parasites cultured without aTc for 96 h before drug pulse (Extended Data Fig. 9a) for FSM (b), AZT (c), DSM265 (d), ATQ (e) and WLL (g). n = 5–7. Statistical significance was determined via two-tailed Mann–Whitney U-tests. *P < 0.05 and **P < 0.01. f, Dose–response curves for ATQ for NF54 parental line with and without aTc, PfMnmA parasites cultured with aTc and PfMnmA parasites cultured without aTc for 96 h before drug pulse, and Dd2 and Dd2_ATQ-R (ATQ resistant, Dd2-CYT1-V259L). The error bars represent s.e.m. n = 6–7 independent biological replicates per parasite line.
The Silent Killer: Malaria's Global Impact
Malaria is more than just a tropical disease; it's a global crisis. Affecting millions and causing over 600,000 deaths annually, the disease's impact is devastating. Plasmodium falciparum, the deadliest of the malaria-causing parasites, has developed resistance to the front-line treatment, artemisinin (ART), especially in regions like Southeast Asia and Africa. ART-based combination therapies (ACTs) are essential in treating uncomplicated malaria, reducing parasite load swiftly when paired with a partner drug to eliminate residual parasites. However, the efficacy of ART is waning, prompting urgent research into the underlying mechanisms of resistance.
Unraveling the Mystery: The Role of tRNA Modification
Researchers have long suspected that the resistance mechanisms of P.
falciparum involve complex biochemical processes. The recent study delves into the cellular process known as transfer ribonucleic acid (tRNA) modification, specifically focusing on a particular modification called thiouridine. This process allows cells to respond quickly to stress by altering RNA molecules, a capability exploited by drug-resistant malaria parasites.
The study reveals that ART-resistant P. falciparum parasites undergo significant changes in their tRNA modifications, particularly in the thiouridine component. These modifications influence the parasite's ability to translate genetic information into proteins, essentially reprogramming their response to the drug. This reprogramming helps the parasites survive the stress induced by ART, thereby contributing to drug resistance.
The Battle Within: Mechanisms of ART Resistance
ART, derived from the sweet wormwood plant, has been a cornerstone in malaria treatment due to its potent parasiticidal activity. However, the emergence of resistance has been linked to mutations in a specific gene coding for kelch-like protein 13 (PfK13). These mutations are associated with delayed parasite clearance and survival during ART treatment.
The research team discovered that ART-resistant parasites exhibit differential hypomodification of mcm5s2U on tRNA, a modification crucial for the translation of lysine, glutamate, and glutamine codons. This alteration leads to changes in protein synthesis, favoring the production of proteins that help the parasite survive drug-induced stress. By comparing ART-sensitive and ART-resistant parasites, the researchers identified a pattern of codon-biased translation, where certain proteins, including PfK13, are regulated in a manner that enhances resistance.
From Cellular Stress to Survival: Epitranscriptomic Pathways
The concept of epitranscriptomics, the study of RNA modifications within cells, plays a pivotal role in understanding drug resistance in malaria. The researchers employed advanced techniques to analyze the epitranscriptomic changes in P. falciparum. By isolating and comparing the RNA of drug-sensitive and drug-resistant parasites, they uncovered how tRNA modifications contribute to the parasite's ability to withstand ART.
One key finding is the role of the unfolded protein response (UPR) and the ubiquitin-proteasome system (UPS) in ART-resistant parasites. These systems help manage cellular stress by degrading damaged proteins and regulating protein synthesis. In ART-resistant parasites, these stress response mechanisms are upregulated, aiding in their survival during drug exposure.
The Genetic Landscape: Codon Bias and Protein Regulation
A fascinating aspect of the study is the role of codon bias in protein synthesis. Codons are sequences of three nucleotides that encode specific amino acids in proteins. The researchers found that ART-resistant parasites exhibit a preference for certain codons, particularly those involving lysine. This bias affects the synthesis of proteins involved in the parasite's stress response and survival.
The study's proteomic analysis revealed that proteins regulated by codon-biased translation include PfK13 and its interactor BIP (Pf3D7_0917900). The levels of these proteins are modulated in response to ART exposure, with K13 levels increasing as ART-resistant parasites exit quiescence, the dormant state they enter to survive drug exposure. This process is akin to bacterial persister cells, which can tolerate stress without genetic changes.
Hypomodification: A Double-Edged Sword
The study's exploration of hypomodification, particularly the hypomodification of mcm5s2U, highlights its dual role in ART resistance and cellular stress response. In ART-resistant parasites, reduced haemoglobin endocytosis, a process essential for nutrient acquisition, leads to lower levels of available amino acids and hypomodification of tRNAs. This, in turn, decelerates protein synthesis and alters metabolic processes, providing a survival advantage under drug-induced stress.
Interestingly, this hypomodification is also observed in other stress conditions, such as heat and oxidative stress. The researchers created a conditional knockdown (cKD) of the terminal enzyme (MnmA) involved in mcm5s2U biosynthesis, confirming the role of hypomodification in ART resistance. These findings suggest that targeting tRNA modifications could be a novel approach to combating drug resistance in malaria.
Beyond Malaria: Broader Implications
The implications of this research extend beyond malaria. The role of tRNA modifications in stress responses and drug resistance is a burgeoning field with potential applications in various diseases, including cancer. Similar mechanisms of tRNA modification reprogramming have been observed in cancer cells, contributing to chemotherapy resistance. Understanding these processes can pave the way for new therapeutic strategies across multiple disciplines.
Future Directions: New Avenues for Drug Development
The discovery of tRNA modification reprogramming as a mechanism of drug resistance in malaria opens new avenues for drug development. Targeting the enzymes involved in these modifications could provide a means to hinder the parasite's ability to adapt to drug-induced stress. This approach could lead to the development of more effective antimalarial drugs and therapies.
The research also emphasizes the importance of epitranscriptomics in understanding disease biology. By exploring the role of RNA modifications in cellular processes, scientists can uncover new targets for therapeutic intervention. The findings from this study provide a foundation for further research into the epitranscriptomic mechanisms underlying drug resistance and other diseases.
Conclusion: A New Dawn in Malaria Research
The fight against malaria is far from over, but the discovery of tRNA thiouridine modification reprogramming offers a glimmer of hope. By unraveling the complex mechanisms of drug resistance, researchers are paving the way for innovative strategies to combat this deadly disease. The collaborative efforts of scientists from SMART, MIT, Columbia University, and NTU Singapore underscore the importance of interdisciplinary research in addressing global health challenges.
As the battle against malaria continues, the insights gained from this study provide a crucial roadmap for developing new therapies and understanding the broader implications of RNA modifications in disease. The resilience of the Plasmodium falciparum parasite may be formidable, but with each scientific breakthrough, humanity inches closer to outsmarting this relentless adversary.
The study findings were published in the peer reviewed journal: Nature Microbiology.
https://www.nature.com/articles/s41564-024-01664-3
For the latest about Malaria, keep on logging to Thailand
Medical News.
