Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 01, 2025 3 months, 1 week, 5 days, 16 minutes ago
Medical News: Recent research highlights an exciting prospect for treating fibrosis in chronic diseases using naturally occurring tryptamines. These substances, long misunderstood as recreational drugs, are now under scientific scrutiny for their potential therapeutic effects. A published study review has shed light on how endogenous tryptamines, particularly N-dimethylated tryptamines, may hold the key to combating fibrosis, a debilitating feature of many chronic conditions.
 Tryptamines as a Potential Weapon Against Fibrosis
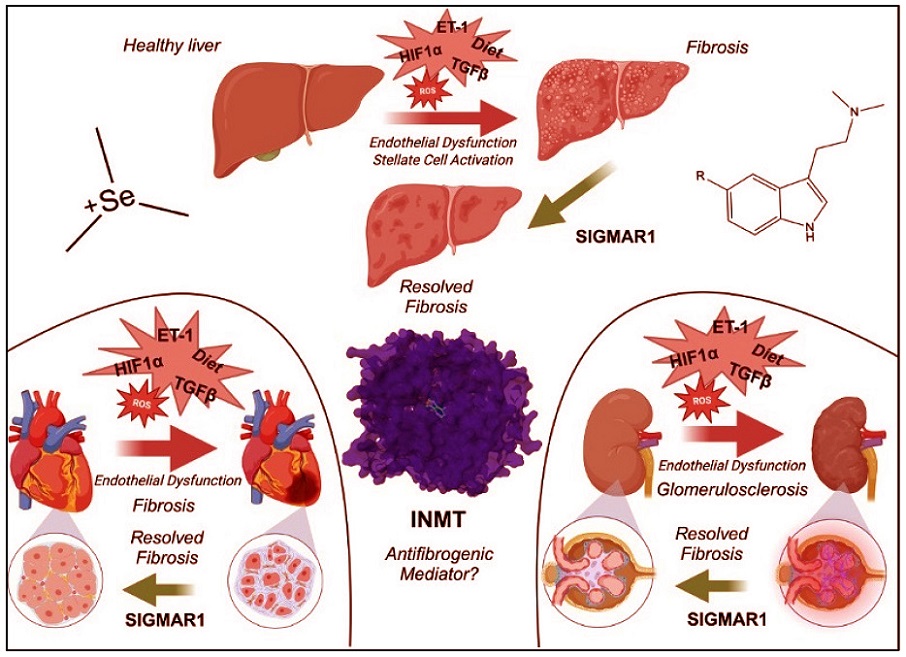
INMT’s potential in mediating fibrogenic diseases through N-methylated tryptamines and selenium metabolism. Red arrows denote insults that promote fibrosis. Green arrows denote resolving of fibrosis through SIGMAR1. PDB: 2A14. Abbreviations: endothelin-1, (ET-1); hypoxia-inducible factor 1-alpha, (HIF1α); indolethylamine-N-methyltransferase, (INMT); reactive oxygen species, (ROS); sigma non-opioid intracellular receptor 1, (SIGMAR1); transforming growth factor beta, (TGFβ).
Tryptamines as a Potential Weapon Against Fibrosis
INMT’s potential in mediating fibrogenic diseases through N-methylated tryptamines and selenium metabolism. Red arrows denote insults that promote fibrosis. Green arrows denote resolving of fibrosis through SIGMAR1. PDB: 2A14. Abbreviations: endothelin-1, (ET-1); hypoxia-inducible factor 1-alpha, (HIF1α); indolethylamine-N-methyltransferase, (INMT); reactive oxygen species, (ROS); sigma non-opioid intracellular receptor 1, (SIGMAR1); transforming growth factor beta, (TGFβ).
This
Medical News report delves into these findings, summarizing the extensive work conducted on the biochemistry and clinical applications of tryptamines.
Fibrosis is the pathological scarring and stiffening of tissue, often associated with conditions like metabolic-associated fatty liver disease (MAFLD), steatohepatitis (MASH), and chronic kidney disease (CKD). In these diseases, the body’s repair mechanisms go awry, leading to excessive collagen deposition and tissue dysfunction. The study in question explores the biochemical pathways involving tryptamines and their potential to disrupt this harmful cycle.
What Are Tryptamines and How Do They Work?
Tryptamines are naturally produced compounds found in humans and other species. These substances include N,N-dimethyltryptamine (DMT), 5-methoxy-DMT, and bufotenine. They are synthesized in the body via an enzyme called indolethylamine-N-methyltransferase (INMT), which uses tryptophan as a precursor. These molecules have a history of being used in traditional ceremonies for their psychoactive effects, but modern science is uncovering their deeper biological significance.
Recent studies show that tryptamines interact with sigma non-opioid intracellular receptor 1 (SIGMAR1), a protein involved in cellular survival and repair. By activating SIGMAR1, tryptamines can enhance the body’s ability to manage stress and inflammation at the cellular level, processes closely tied to fibrosis.
Linking Tryptamines and Fibrosis
Fibrosis typically arises from chronic inflammation and endothelial damage, which triggers a cascade of events leading to excessive scar tissue formation. In conditions like MAFLD, liver sinusoidal endothelial cells (LSECs) and hepatic stellate cells (HSCs) play a central role. When these cells are damaged, they release signals that activate fibroblasts to produce collagen. This overproduction results in the stiffening and loss of function in the affect
ed organ.
Tryptamines, through their interaction with SIGMAR1, may intervene in this process. Preclinical studies have demonstrated that these molecules can reduce oxidative stress, inflammation, and cellular damage - all of which contribute to fibrosis. By stabilizing cellular environments and modulating stress responses, tryptamines may help curb the fibrotic cycle.
The Microbiome Connection
Interestingly, the gut microbiome also appears to influence tryptamine production and activity. Certain gut bacteria can metabolize tryptophan into tryptamine and other related compounds. These microbiota-derived metabolites have been shown to impact systemic inflammation and lipid metabolism, both of which are crucial in conditions like MAFLD and CKD. Researchers hypothesize that modulating the gut microbiome could enhance the body’s natural production of therapeutic tryptamines.
Key Findings from the Study
The study under discussion provides several groundbreaking insights:
-Endothelial Protection: Tryptamines were shown to protect endothelial cells, which line blood vessels, from damage caused by oxidative stress and hypoxia (low oxygen conditions).
-Anti-inflammatory Effects: By activating SIGMAR1, tryptamines reduced the expression of pro-inflammatory genes and proteins, mitigating the inflammatory responses that often precede fibrosis.
-Reduction in Fibroblast Activation: Tryptamines interfered with the signaling pathways that activate fibroblasts, the cells responsible for collagen production in fibrosis.
-Potential Antioxidant Role: Due to their chemical structure, tryptamines may act as short-lived antioxidants, neutralizing reactive oxygen species that contribute to cellular damage and inflammation.
-Gut-Liver Axis: The study emphasized the role of microbiota in producing and regulating tryptamine levels, suggesting that dietary and probiotic interventions could complement therapeutic strategies.
Therapeutic Implications and Challenges
While the findings are promising, there are challenges to overcome before tryptamines can be widely used in clinical settings. One major hurdle is their psychoactive effects, which could limit their applicability. However, researchers are exploring ways to isolate the therapeutic benefits of tryptamines from their mind-altering properties. Liposomal delivery systems and targeted drug designs are being tested to enhance the efficacy and safety of tryptamine-based therapies.
Broader Applications
Beyond fibrosis, tryptamines have shown potential in addressing other chronic conditions. For example, they have been implicated in neuroprotection, offering hope for diseases like Alzheimer’s. Their ability to modulate stress responses and repair cellular damage makes them attractive candidates for a range of inflammatory and degenerative diseases.
Conclusion
The role of tryptamines in fibrosis and chronic disease management represents a frontier in medical research. By targeting fundamental pathways like SIGMAR1 activation and oxidative stress reduction, these compounds offer a novel approach to treating conditions that currently have limited therapeutic options. Future studies should focus on refining their delivery mechanisms and understanding their broader systemic effects.
The study findings were published in the peer-reviewed journal: Livers.
https://www.mdpi.com/2673-4389/4/4/43
For the latest on Fibrosis, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/new-hopes-for-treating-lung-fibrosis
https://www.thailandmedical.news/news/alpinia-officinarum-extract-as-a-potential-treatment-for-lung-fibrosis-
https://www.thailandmedical.news/news/how-calcium-signals-could-unlock-better-treatments-for-heart-and-lung-fibrosis
https://www.thailandmedical.news/news/natural-compounds-show-promise-for-treating-intestinal-fibrosis
https://www.thailandmedical.news/news/the-phytochemical-isorhamnetin-could-be-harnessed-to-treat-kidney-fibrosis
