Type I Interferon Autoantibodies In COVID-19 Cause Immune Dysregulation And Contribute To Disease Severity
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 04, 2024 1 year, 1 month, 2 weeks, 4 hours, 19 minutes ago
COVID-19 News: The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has presented unique challenges due to the variability in disease severity among infected individuals. Researchers at Karolinska Institutet and Karolinska University Hospital in Sweden have delved into the intricate interplay between the immune system and severe COVID-19, uncovering a significant association between the presence of autoantibodies against type I interferons (aIFN-Abs) and the severity of the disease. This
COVID-19 News report explores their findings, shedding light on the impact of aIFN-Abs on various facets of the immune response.
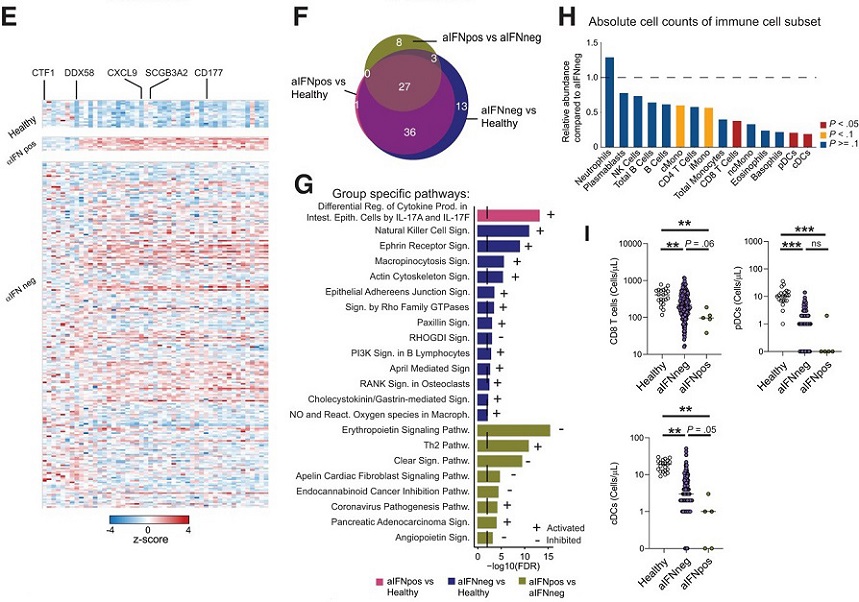 Autoantibodies against type 1 IFN modulate the cellular immune compartment.
E, Heatmap displaying z scores of the differentially expressed genes between aIFNpos and aIFNneg patients, for healthy controls, and aIFNpos and aIFNneg patients. F, Venn diagram displaying shared and distinct pathways calculated with the IPA for the different comparisons of the 3 groups. G, Overview of the specific IPA pathways in (F) for the indicated comparisons. H, Ratio of average absolute lymphocyte counts when comparing aIFNpos (n = 5) to aIFNneg (n = 174) patients in the acute phase of COVID-19. Groups were compared with Mann-Whitney test; blue P > = .1, yellow P < .1, red P < .05.
Understanding the Importance of Type I Interferons
Autoantibodies against type 1 IFN modulate the cellular immune compartment.
E, Heatmap displaying z scores of the differentially expressed genes between aIFNpos and aIFNneg patients, for healthy controls, and aIFNpos and aIFNneg patients. F, Venn diagram displaying shared and distinct pathways calculated with the IPA for the different comparisons of the 3 groups. G, Overview of the specific IPA pathways in (F) for the indicated comparisons. H, Ratio of average absolute lymphocyte counts when comparing aIFNpos (n = 5) to aIFNneg (n = 174) patients in the acute phase of COVID-19. Groups were compared with Mann-Whitney test; blue P > = .1, yellow P < .1, red P < .05.
Understanding the Importance of Type I Interferons
Type I interferons (IFNs) play a crucial role in the body's defense against viral infections, orchestrating both local and systemic antiviral responses. However, in severe COVID-19 cases, there is evidence of a dysregulated type I IFN pathway. Patients with critical COVID-19 and other severe viral diseases often exhibit inborn errors in this pathway, pointing to its significance in controlling viral infections. Autoantibodies against type I IFNs, particularly IFN-α, known as aIFN-Abs, have been identified in up to 15% of patients with critical COVID-19, contributing to increased morbidity and mortality.
Prevalence and Dynamics of aIFN-Abs in COVID-19
The research conducted at Karolinska Institutet involved analyzing a cohort of 257 COVID-19 patients, focusing on the presence and dynamics of aIFN-Abs. The study revealed that aIFN-Abs were more prevalent in patients with critical COVID-19, particularly those requiring intensive care unit (ICU) treatment, compared to individuals with severe disease. Strikingly, these autoantibodies were rarely detected in other viral and bacterial infections studied, emphasizing their specific association with severe COVID-19.
Impact on Mortality and Clinical Characteristics
Patients with aIFN-Abs exhibited higher mortality rates, underscoring the potential prognostic value of these autoantibodies. The study found that patient characteristics such as age, sex, comorbidity, and body mass i
ndex were comparable between those with and without aIFN-Abs, indicating that the presence of these autoantibodies was a distinct factor influencing disease severity.
Soluble Proteome Alterations and Inflammatory Responses
To gain insights into the effects of aIFN-Abs on the immune system, the researchers explored alterations in the soluble proteome, encompassing 1463 proteins, in patients with severe COVID-19. While both aIFNpos and aIFNneg patients exhibited a significant inflammatory response, only minor differences were observed between the two groups. Notably, pathways related to Th2 response and "coronavirus pathology" were enriched in aIFNpos patients, indicating a nuanced impact on immune activation pathways.
Cellular Immune Alterations
The study delved into the cellular immune compartment to investigate the impact of aIFN-Abs on immune cell composition. A significant reduction in absolute immune cell counts, particularly CD8 T cells and dendritic cells, was observed in aIFNpos patients compared to aIFNneg patients. Importantly, the T-cell phenotype and function remained largely unaltered, suggesting a predominant loss of immune cells in the periphery.
Humoral Immunity and Antibody Responses
Considering the influence of type I IFNs on B cells and antibody production, the researchers assessed the humoral immune response in patients with aIFN-Abs. Surprisingly, the B-cell phenotype and B-cell receptor repertoire remained stable. Moreover, aIFNpos patients exhibited efficient antibody responses against SARS-CoV-2 and other viruses, emphasizing that despite impaired type I IFN responses, humoral immunity remained intact.
Longitudinal Dynamics and Implications
The study also investigated the temporal dynamics of aIFN-Abs, revealing their stability over a prolonged period. Longitudinal assessments indicated a decline in aIFN-Ab levels over time, but some patients maintained persistently high levels even a year after acute COVID-19. The researchers concluded that aIFN-Abs are specific to certain infections, notably COVID-19, and do not lead to broad inhibition of peripheral immune responses.
Conclusion and Future Perspectives
In summary, the research from Karolinska Institutet provides valuable insights into the intricate relationship between aIFN-Abs and severe COVID-19. While these autoantibodies associate with alterations in the cellular immune compartment, they do not significantly impact the development of adaptive immunity or humoral responses. Future studies should explore the local effects of aIFN-Abs at the site of infection and their potential implications for respiratory infections beyond COVID-19. Understanding the role of aIFN-Abs in immune dysregulation opens avenues for targeted interventions and personalized treatment strategies in severe viral infections.
The study findings were published in the peer reviewed journal: The Journal of Infectious Diseases.
https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiae036/7616238
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
