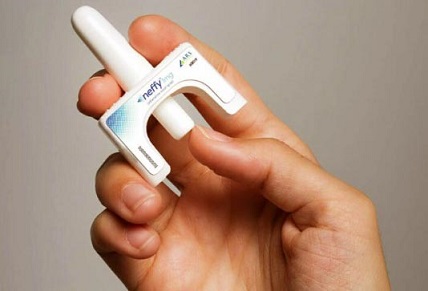
U.S. FDA approves Neffy, the first nasal epinephrine spray for treating severe allergic reactions
Nikhil Prasad Fact checked by:Thailand Medical News Team Aug 15, 2024 8 months, 1 week, 4 days, 2 hours, 59 minutes ago
Med News: In a groundbreaking development for allergy sufferers, the U.S. Food and Drug Administration (FDA) has approved the first-ever epinephrine nasal spray, known as Neffy, for treating severe allergic reactions, including life-threatening anaphylaxis.
https://www.fda.gov/news-events/press-announcements/fda-approves-first-nasal-spray-treatment-anaphylaxis
 U.S. FDA approves Neffy, the first nasal epinephrine spray for treating severe allergic reactions
U.S. FDA approves Neffy, the first nasal epinephrine spray for treating severe allergic reactions
This new treatment option, developed by ARS Pharmaceuticals, represents a significant advancement in managing allergic emergencies, offering a needle-free alternative to the traditional epinephrine injections that have long been the standard.
A New Hope for Allergy Sufferers
For millions of Americans who live with severe allergies, the fear of anaphylaxis is a constant concern. Anaphylaxis is a severe and potentially fatal allergic reaction that can be triggered by a variety of factors, including food, medication, and insect stings. Symptoms can escalate rapidly, leading to difficulty breathing, a drop in blood pressure, and loss of consciousness. The only effective treatment has historically been an injection of epinephrine, typically administered via an auto-injector like the widely known EpiPen.
However, the need for injections has posed a significant barrier for many patients. Needle phobia, lack of portability, and concerns about safety and proper administration have led to hesitation and delays in using these life-saving devices. ARS Pharmaceuticals, the manufacturer of Neffy, recognized these challenges and sought to provide a solution that would make treatment more accessible and user-friendly.
How Neffy Works
Neffy is designed to be a simple, one-step solution for delivering epinephrine. The nasal spray is administered as a single dose into one nostril, and in cases where symptoms do not improve or worsen, a second dose can be administered using a new spray in the same nostril. This ease of use is expected to encourage more timely treatment during emergencies, potentially saving lives.
The U.S. FDA's approval of Neffy was based on data from four studies involving 175 healthy adults. These studies compared the blood concentrations of epinephrine following the administration of Neffy to those achieved with traditional epinephrine injection products. The results showed that Neffy could achieve comparable epinephrine levels and physiological responses, such as increases in blood pressure and heart rate, which are critical in counteracting the effects of anaphylaxis.
Moreover, a study conducted on children weighing more than 66 pounds confirmed that the nasal spray was effective in this age group, with blood concentrations of epinephrine similar to those seen in adults. This broadens the applicability of Neffy, making it a viable option for both adults and children.
Addressing Barriers to Treatment
One of the most significant advantages of Neffy is its potent
ial to overcome the barriers that have historically prevented or delayed treatment with epinephrine injections. The fear of needles, particularly among children, is a well-documented issue that has led many to avoid or postpone the use of auto-injectors, even in emergency situations. By offering a needle-free alternative, Neffy is expected to increase compliance and reduce the risks associated with delayed treatment.
ARS Pharmaceuticals has emphasized the importance of providing a treatment option that is both effective and easy to use. The company told
Med News journalists that there are approximately 40 million people in the U.S. who have type 1 allergic reactions, yet only 3.2 million of them have a prescription for an epinephrine injection pen. The introduction of Neffy could significantly increase the number of individuals who are adequately prepared to handle allergic emergencies.
Considerations and Side Effects
While Neffy presents a promising alternative to traditional epinephrine injections, there are certain considerations that patients and healthcare providers should keep in mind. The FDA has advised that individuals with certain nasal conditions, such as polyps or a history of nasal surgery, should consult with a healthcare professional before using Neffy, as these conditions could affect the absorption of the medication.
Additionally, Neffy may not be suitable for individuals with specific coexisting conditions or those who are allergic to sulfites. The most commonly reported side effects include nasal irritation, throat irritation, headaches, and a jittery sensation, all of which are associated with the effects of epinephrine itself. Despite these potential discomforts, the ease of administration and the ability to act quickly in an emergency may outweigh these minor drawbacks for many users.
Availability and Cost
Neffy is expected to be available to consumers within eight weeks of its FDA approval. ARS Pharmaceuticals has announced that for patients with commercial insurance, Neffy will cost $25 for two single-use devices. For those without insurance or whose insurance does not cover Neffy, the company will offer it at a cash price of $199. Additionally, ARS has committed to providing Neffy at no cost to uninsured patients who have exhausted all other options, ensuring that the life-saving medication is accessible to those who need it most.
A New Era in Allergy Treatment
The FDA's approval of Neffy marks a significant milestone in the treatment of severe allergic reactions. By offering a non-invasive, needle-free method of delivering life-saving medication, Neffy is expected to transform the way healthcare providers manage anaphylaxis and other serious allergic reactions. This approval, which came after an initial delay pending additional safety data, highlights the FDA's commitment to ensuring that new treatment options are both effective and safe for widespread use.
As ARS Pharmaceuticals prepares to bring Neffy to market, the healthcare community and allergy sufferers alike are looking forward to the positive impact this new treatment option could have. The introduction of Neffy is not just a step forward in allergy treatment; it represents a broader shift towards patient-centered care that prioritizes accessibility, ease of use, and timely intervention.
For the latest
Med News updates and other developments in allergy treatment, visit Thailand Medical News regularly.
Read Also:
https://www.thailandmedical.news/news/sanofi-s-children-allergy-medications-recalled-in-india-due-to-possible-microbiological-contamination
https://www.thailandmedical.news/news/pirfenidone-brings-hope-for-fibrotic-interstitial-lung-diseases
