U.S NIH’s And U.S. Army’s NeuroCOVID Study Shows That SARS-CoV-2 Infections Causes Serious Damage To Microvessels In the Brain!
Source; Medical News - NeuroCOVID Aug 28, 2022 3 years, 4 months, 1 day, 9 hours, 57 minutes ago
NeuroCOVID: A study led by researchers from the National Institute of Neurological Disorders and Stroke - U.S. National Institutes of Health and the U.S. Army’s Uniformed Services University of the Health Sciences and The Joint Pathology Center at the Defense Health Agency in Maryland have found that SARS-CoV-2 infections causes serious damage to the microvessels of the brain.
To date, the underlying mechanisms by which SARS-CoV-2 infections lead to a variety of acute and long-term neurological manifestations remains obscure.
The study team aimed to characterize the neuropathological changes in patients with coronavirus disease 2019 and determine the underlying pathophysiological mechanisms.
The study team in this autopsy study of the brain characterized the vascular pathology, the neuroinflammatory changes and cellular and humoral immune responses by immunohistochemistry.
The research subjects were patients who died during the first wave of the pandemic from March to July 2020. All patients were adults who died after a short duration of the infection.
It was noted that some had died suddenly with minimal respiratory involvement.
For the study, infection with SARS-CoV-2 was confirmed on ante-mortem or post-mortem testing.
Subsequent descriptive analysis of the pathological changes and quantitative analyses of the infiltrates and vascular changes were performed.
The study findings showed that all the research subjects had multifocal vascular damage as determined by leakage of serum proteins into the brain parenchyma. This was accompanied by widespread endothelial cell activation.
Furthermore, platelet aggregates and microthrombi were found adherent to the endothelial cells along vascular lumina. Immune complexes with activation of the classical complement pathway were found on the endothelial cells and platelets. Perivascular infiltrates consisted of predominantly macrophages and some CD8+ T cells. Only rare CD4+ T cells and CD20+ B cells were present.
Astrogliosis was also prominent in the perivascular regions. Microglial nodules were predominant in the hindbrain, which were associated with focal neuronal loss and neuronophagia. (Astrogliosis is a defense mechanism to minimize and repair the initial damage after CNS injuries and is characterized by remarkable molecular, cellular, and functional alterations in glial cells, especially in reactive astrocytes.)
Corresponding author, Dr Avindra Nath told Thailand
Medical News, “Antibody-mediated cytotoxicity directed against the endothelial cells is the most likely initiating event that leads to vascular leakage, platelet aggregation, neuroinflammation and neuronal injury.”
The study findings indicate that therapeutic modalities directed against immune complexes should be considered.
The study findings were published in the peer reviewed journal: Brain.
https://academic.oup.com/brain/article/145/7/2555/6621999
;
Already in the early part of the start of the COVID-19 pandemic, it was already found that SARS-CoV-2 infections also brought about a variety of neurological issues which were collected group under the term
NeuroCOVID.
Numerous early studies described signs of vascular abnormalities in the brains of severely ill COVID-19 patients that were verified by immunohistology in post-mortem tissues.
As a result of increasing evidence, it is now accepted that SARS-CoV-2 infections contribute to damage of the vasculature of various organs and that these effects may contribute to organ dysfunction.
But until now, it was still unclear exactly what happens to the brain microvessels and which mechanisms are involved in order to provide an explanation for why COVID-19 includes neurological symptoms.
The study team authors used additional techniques to characterize in more detail vascular and immunological features of microvessels in the brain.
Interestingly, compared to controls, the brain tissue of patients who died from COVID-19 showed a dramatic increase in the extravasation of serum proteins, especially fibrinogen, indicating a disturbed blood–brain barrier.
Also, platelet accumulation in combination with augmented expression of platelet endothelial cell adhesion molecule 1 (PECAM-1), as well as increased tissue factor and von Willebrand factor was observed in COVID-19 patients, suggesting that activation of the coagulation system in the brain vasculature most likely leads to occlusion and damage to small vessels.
The findings correlated with the study team earlier study findings of evidence of injured cerebral blood vessels obtained using MRI and also that of another published study from Harvard researchers.
https://pubmed.ncbi.nlm.nih.gov/33378608/
https://pubmed.ncbi.nlm.nih.gov/33497950/
Importantly, the study team detected immune complexes using multiplex fluorescence microscopy at the vascular wall. These complexes were positive for IgG/IgM antibodies and co-localized with complement factors.
A past study had showed that complement activation in COVID-19, but not directly in the brain.
https://pubmed.ncbi.nlm.nih.gov/32726800/
The formation of the membrane attack complex (MAC), consisting of several activated complement factors can contribute to the endothelial cell death that was detected in another reserach.
https://pubmed.ncbi.nlm.nih.gov/34675436/
It was found that an activated endothelium and more permeable blood–brain barrier could result in infiltration of immune cells as seen in other neurological diseases.
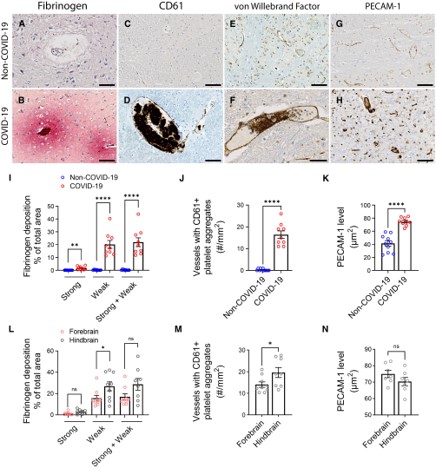 Microvascular injury and thrombus formation. Immunostaining for (A and B) fibrinogen, (C and D) CD61 for activated platelets, (E and F) vWF and (G and H) PECAM-1 shows absence or minimal staining in the non-COVID-19 brain but in the COVID-19 patients, there was (B) perivascular leakage of fibrinogen, (D) platelet aggregates, (F) thrombi and (H) increased PECAM-1 on endothelial cells. Significantly greater (I) leakage of fibrinogen (**P = 0.0011, ****P < 0.0001) and (J) blood vessels with platelet aggregates were present in the COVID-19 brains (****P < 0.0001). (K) PECAM-1 was significantly increased in the brains of COVID-19 patients compared to non-COVID-19 controls (****P < 0.0001). (L–N) Dots represent the average of individual values in the brain regions that make up the forebrain or hindbrain of a patient. The forebrain includes the cerebrum, basal ganglia and thalamus. The hindbrain contains the pons, medulla and cerebellum. (L) Strong fibrinogen deposition was equally distributed in different regions of the brain, while weak deposition was more prevalent in the hindbrain compared to the forebrain (*P = 0.04). (M) There were more thrombi in the hindbrain compared to the forebrain of the COVID-19 patients (*P = 0.04). (N) PECAM-1 immunostaining was equally distributed in different brain regions of the COVID-19 patients. Data represents mean ± SEM. Scale bars = 100 µm.
Microvascular injury and thrombus formation. Immunostaining for (A and B) fibrinogen, (C and D) CD61 for activated platelets, (E and F) vWF and (G and H) PECAM-1 shows absence or minimal staining in the non-COVID-19 brain but in the COVID-19 patients, there was (B) perivascular leakage of fibrinogen, (D) platelet aggregates, (F) thrombi and (H) increased PECAM-1 on endothelial cells. Significantly greater (I) leakage of fibrinogen (**P = 0.0011, ****P < 0.0001) and (J) blood vessels with platelet aggregates were present in the COVID-19 brains (****P < 0.0001). (K) PECAM-1 was significantly increased in the brains of COVID-19 patients compared to non-COVID-19 controls (****P < 0.0001). (L–N) Dots represent the average of individual values in the brain regions that make up the forebrain or hindbrain of a patient. The forebrain includes the cerebrum, basal ganglia and thalamus. The hindbrain contains the pons, medulla and cerebellum. (L) Strong fibrinogen deposition was equally distributed in different regions of the brain, while weak deposition was more prevalent in the hindbrain compared to the forebrain (*P = 0.04). (M) There were more thrombi in the hindbrain compared to the forebrain of the COVID-19 patients (*P = 0.04). (N) PECAM-1 immunostaining was equally distributed in different brain regions of the COVID-19 patients. Data represents mean ± SEM. Scale bars = 100 µm.
The study findings of the present research provided data supporting the infiltration of CD3- or CD8-positive T cells and CD68-positive macrophages. These infiltrates were present almost exclusively in the perivascular space and not in the brain parenchyma, indicating indirect effects, if any, on cells like neurons and glia.
The neuropathological abnormalities in patients with severe COVID-19 were most prominent in the hindbrain, suggesting a more susceptible vasculature or increased entry of SARS-CoV-2 in this region.
https://pubmed.ncbi.nlm.nih.gov/33031735/
The current study team even found morphological signs of neuronophagia in the hindbrain, suggesting neuronal cell death and phagocytosis by microglia.
The study team also used an elaborate technique to examine spatial transcriptomics in the hindbrain, focusing on regions enriched in the endothelial marker PECAM-1 or the immune cell marker CD45, thereby generating a dataset that could be useful for future studies. Notably, the expression of several genes encoding factors involved in perfusion regulation such as eNOS or Kir2.1 was differentially regulated in control versus COVID-19 patients.
The study findings provides an important overview of what is going on in the brains of COVID-19 patients. However, the initial trigger of vascular damage remains hypothetical.
The study team says that immunoglobulin complexes stimulate the classical complement cascade, activate endothelial cells, and subsequently induce blood–brain barrier leakage and immune cell infiltration.
Past studies have shown that autoantibodies were detected not only in the blood and CSF of severely ill COVID-19 patients, but also in patients suffering from post-acute sequelae of COVID-19.
https://pubmed.ncbi.nlm.nih.gov/33330894/
https://pubmed.ncbi.nlm.nih.gov/33880442/
These autoantibodies were able to bind directly to vascular antigens, and may even show agonist activity. Again however, it is unclear whether endothelial cells in the brain are directly affected.
The new study findings showing immunoglobulin complexes bound to the vascular wall may be a hint in this direction.
Aside from autoantibodies, the endothelium in the brain can be activated by cytokines like interleukins or TNF that are known to be released in large quantities during acute COVID-19. These cytokines act directly on endothelial cells, leading to adhesion of blood cells and opening of the blood–brain barrier with subsequent immune cell infiltration.
Yet another possibility for how SARS-CoV-2 could affect microvessels in the brain is via a direct effect of the virus on the endothelium.
Although the present study team did not find signs of direct viral infection of brain tissue, other past studies have detected viral particles in the brains of patients and in vascular cells. An explanation for the rare detection of SARS-CoV-2 could be the rapid induction of cell death in infected endothelial cells.
A past study had shown that macrophages in the lung undergo cell death after SARS-CoV-2 infection, explaining the depletion of these cells in the respiratory tract.
https://www.nature.com/articles/s41586-022-04802-1
The death of pulmonary macrophages was secondary to an inflammasome activation leading to the release of cytokines and the induction of a hyperinflammatory state. Also, another interesting finding in that study was that induced cell death prevented SARS-CoV-2 replication in infected cells and therefore viral spreading. In principle, one could imagine a similar mechanism applying to endothelial cells in the brain. Viral infection and expression of SARS-CoV-2 proteins has been shown to induce cell death in the brain endothelium, and the same mechanisms trigger a neuroinflammatory state. As a side effect, the death of endothelial cells will activate the vasculature in the brain, leading to a leaky blood–brain barrier, adhesion of plasma proteins, and infiltration of immune cells.
Hence, examining the vasculature and its interactions with the immune system is thus proving key to understanding organ dysfunction in COVID-19. These interactions may be responsible for severe acute illness but also for the post-acute sequelae of COVID-19.
https://pubmed.ncbi.nlm.nih.gov/35271343/
Importantly, hypoperfusion and immune cell infiltration as well as glial activation in the brain parenchyma may trigger long-lasting neurological symptoms in COVID-19 patients.
The study findings show that SARS-CoV-2 infection impairs vascular function, and provides valuable insights into the molecular players involved in the interaction between the vasculature and the immune system. Future efforts to find an effective therapy for the acute and post-acute phases of COVID-19 should consider targeting the cerebrovascular effects of the disease.
For the latest on
NeuroCOVID, keep on logging to Thailand
Medical News.

