U.S. NIH Study Shows That Omicron Spike Proteins Confers Enhanced Infectivity And Interferon Resistance To SARS-CoV-2 In Human Nasal Tissue!
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 01, 2024 1 year, 2 months, 2 weeks, 16 hours, 38 minutes ago
COVID-19 News: The relentless evolution of the SARS-CoV-2 virus has ushered in the era of the Omicron variant, a paradigm shift in the dynamics of the ongoing COVID-19 pandemic. Originating in the wake of widespread vaccination campaigns, Omicron swiftly supplanted its predecessors, notably the Delta variant, and imposed its dominance on a global scale. A groundbreaking study covered in this
COVID-19 News report, conducted by the U.S. National Institutes of Health (NIH) sheds light on the molecular intricacies of Omicron's behavior within human nasal tissue, revealing a nuanced interplay of factors contributing to its enhanced infectivity and resistance to interferon-induced antiviral defenses.
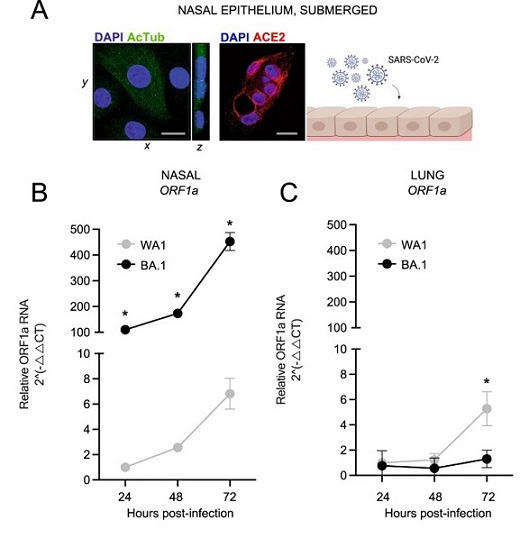 Omicron BA.1 exhibits superior replicative fitness relative to early SARS-CoV-2 and Delta in primary human nasal epithelial cells.
Omicron BA.1 exhibits superior replicative fitness relative to early SARS-CoV-2 and Delta in primary human nasal epithelial cells.
A Primary human nasal epithelial cells (pooled from three human donors) were cultured as undifferentiated, submerged monolayers and challenged with SARS-CoV-2. Cells were fixed and permeabilized for confocal immunofluorescence microscopy. Acetylated tubulin levels were determined by anti-AcTub immunofluorescence, ACE2 levels were determined by anti-ACE2 immunofluorescence, and DAPI was used to stain nuclei. 3D reconstructions of xy and yz fields are shown. Scale bars = 10 µm. Cartoon made with Biorender.com. B Primary human nasal epithelial cells (pooled from three donors) or C human small airway (lung) epithelial cells (pooled from three donors) were challenged with replication-competent SARS-CoV-2 WA1 or Omicron BA.1 at an MOI of 0.05. Total cellular RNA was extracted, and viral ORF1a was quantified by RT-qPCR at the indicated time points. Relative viral RNA abundance compared to actin was determined by the 2(−ΔΔCT) method. ORF1a abundance of WA1 at 24 h post inoculation was set to 1
Omicron's Unique Spike: A Molecular Maestro in Enhanced Entry
At the heart of Omicron's enhanced infectivity lies its Spike protein, adorned with a myriad of mutations that distinguish it from earlier SARS-CoV-2 variants. In a meticulous exploration, researchers from the National Cancer Institute in Frederick, Maryland-USA, and the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland-USA, utilized recombinant forms of SARS-CoV-2 and primary human nasal epithelial cells cultured at the liquid-air interface. This cutting-edge approach unveiled Omicron's unparalleled ability to infiltrate nasal tissue with greater efficiency than its predecessor, the Delta variant.
Contrary to earlier variants, Omicron's Spike exhibits a remarkable independence from serine transmembrane proteases. Instead, it relies on metalloproteinases to catalyze membrane fusion, orchestrating a unique entry mechanism into nasal cells. Unlike the traditional pathway involving serine transmembrane proteases like TMPRSS2, Omicron's unconventional reliance on
metalloproteinases represents a significant departure from established norms in viral entry dynamics.
Omicron's Entry Pathway: A Symphony of Metalloproteinases
Delving deeper into Omicron's entry pathway, the study identified the crucial role of metalloproteinases from the MMP/ADAM families. This unconventional route not only sets Omicron apart from its viral ancestors but also grants it a dual advantage. While facilitating entry into nasal cells, the metalloproteinase-mediated pathway concurrently renders Omicron resistant to the constitutive and interferon-induced antiviral factors that typically restrict SARS-CoV-2 entry post-attachment.
Comparative Analysis of Omicron's Infectivity
To gauge the extent of Omicron's impact, the researchers conducted a meticulous comparison between Omicron variants (BA.1 and BA.2) and early SARS-CoV-2, the D614G variant, and the Delta variant. Utilizing a state-of-the-art primary human nasal tissue culture model, Omicron consistently demonstrated significantly heightened infectivity in nasal epithelia. The study attributed this augmented infectivity to specific mutations within the Spike protein of Omicron, underscoring the pivotal role of these genetic alterations in enhancing binding and entry into nasal cells.
Metalloproteinase-Mediated Entry - A Double-Edged Sword
While metalloproteinases emerged as facilitators of Omicron's entry, their involvement also introduced a dual function. Beyond enabling entry, the metalloproteinase-mediated pathway rendered Omicron resistant to type-I and type-III interferons, presenting a complex challenge for the host's innate immune response. This dual functionality not only contributes to Omicron's heightened transmissibility but also poses a formidable obstacle for the host's immune defenses, allowing Omicron to establish infection more effectively in the nasal epithelium.
Unraveling Omicron's Interactions with Cellular Factors
The study meticulously explored Omicron's interactions with cellular factors crucial for viral entry. The increased net positive charge of Omicron's Spike protein endowed it with a superior ability to adhere to nasal epithelial cells, regardless of the presence of cilia. This unique feature prompted speculation that mutations in Omicron Spike promote an extended residence time at the cell surface, influencing its dependence on specific cellular proteases for cleavage and fusion.
IFN Resistance - A Strategic Advantage
One of the most intriguing aspects uncovered by the study was Omicron's resistance to interferons, providing a strategic advantage in evading the host's antiviral defenses. In contrast to earlier variants, Omicron exhibited a reduced capacity to interfere with type-I and type-III interferon production and signaling. Importantly, this resistance was not attributed to Omicron's ability to interfere with interferon pathways but rather to its Spike-mediated entry process, providing a plausible explanation for Omicron's decreased antagonism against interferon pathways compared to previous variants.
Implications for Future Therapeutics
The study's findings hold significant implications for the ongoing development of antiviral therapeutics, especially type-I and type-III interferons. Omicron's heightened resistance to these therapeutic interventions underscores the need for tailored approaches to combat infections caused by this variant. Clinical trials involving IFN-lambda, for instance, have yielded conflicting results, necessitating further research to determine the efficacy of such treatments against Omicron and potential future variants.
Conclusion
In conclusion, the NIH study offers a comprehensive understanding of Omicron's modus operandi within the human nasal tissue, unraveling the intricacies of its enhanced infectivity and interferon resistance. The unique entry pathway mediated by metalloproteinases, coupled with Spike mutations promoting increased adhesion, provides a multi-faceted explanation for Omicron's ability to outcompete and displace earlier variants. As the global scientific community strives to stay one step ahead of the ever-evolving SARS-CoV-2, the insights gained from this study pave the way for targeted therapeutic strategies and a deeper comprehension of the selective forces driving the emergence and dominance of Omicron.
The study findings were published in the peer reviewed journal: Nature Communications.
https://www.nature.com/articles/s41467-024-45075-8
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
