UK Study Shows That SARS-CoV-2 Spike S1 Glycoprotein Is A TLR4 Agonist, Upregulates ACE2 And Induces Pro-Inflammatory M1 Macrophage Polarization
Source: COVID-19 Immunology Aug 17, 2021 4 years, 3 months, 3 weeks, 5 days, 16 hours, 35 minutes ago
A new study by researchers from King’s College London-UK has shown that the SARS-CoV-2 coronavirus spike S1 glycoprotein is a TLR4 (toll-like receptor 4) agonist that also upregulates ACE2receptors and induces pro-inflammatory M1 (Monocyte) macrophage polarization.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2021.08.11.455921v1
At present the world is currently witnessing the start of another surge caused by the dominant prevailing Delta variant and so far the COVID-19 pandemic has infected over 208 million individuals and caused the deaths of more than 4.38 million individuals according to official statistics. According to the U.S. CDC, the real figures can be eight to ten-fold!
The novel SARS-CoV-2 coronavirus has a spike glycoprotein on its outer envelope, mediating the interaction between the virus and the human receptor, namely, angiotensin-converting enzyme 2 (ACE2) and very often the virus gains entry into the host cell.
TLR4 or toll-like receptor 4 is a protein in humans that is encoded by the TLR4 gene. TLR4 is a transmembrane protein, member of the toll-like receptor family, which belongs to the pattern recognition receptor (PRR) family. Its activation leads to an intracellular signaling pathway NF-κB and inflammatory cytokine production which is responsible for activating the innate immune system.
TRL4 expressing cells are myeloid (erythrocytes, granulocytes, macrophages) rather than lymphoid (T-cells, B-cells, NK cells). Most myeloid cells also express high levels of CD14, which facilitates activation of TLR4 by LPS. It is most well-known for recognizing lipopolysaccharide (LPS), a component present in many Gram-negative bacteria (e.g. Neisseria spp.) and selected Gram-positive bacteria. Its ligands also include several viral proteins, polysaccharide, and a variety of endogenous proteins such as low-density lipoprotein, beta-defensins, and heat shock protein. It should also be noted that Palmitic acid is also a TLR4 agonist.
The primary function of this receptor protein is to identify specific pathogenic (bacterial, viral, fungal, and parasite) components, known as pathogen-associated-molecular-patterns (PAMPs), and trigger cellular and systematic immune responses.
It has been found that activation of TLR4 is associated with the secretion of chemokines and pro-inflammatory cytokines, which cause inflammation. In addition, activation of TLR4 also results in the secretion of type 1 interferons (e.g., b-interferon), whose primary role is to alert neighboring cells about viral infection and inhibit viral replication.
Interestingly TLR4 is present in both cell surfaces and endosomes. TLR4 is the only TLR that has canonical and alternative downstream signaling pathways: (a) a canonical MyD88-dependent pathway involves the release of pro-inflammatory cytokines and chemokines and (b) the TRIF/TRAM-dependent endosomal pathway results in the
secretion of type I interferons and some anti-inflammatory cytokines. Both these pathways are critical because they help regulate the immune response.
Past studies have revealed that expression of ACE2 in the lung epithelial cells is low. However, SARS-CoV-2 is a respiratory disease, which causes hyperinflammation, known as cytokine storm, in severely infected individuals.
However such a contradiction has raised a question regarding the pathophysiology of SARS-CoV-2. A previous piece of research associated with COVID-19 infection revealed SARS-CoV-2 gains entry into the host cell by activating the TLR4 pathway at the early stage of infection.
The study shows that the spike glycoprotein binds and activates TLR4, a transmembrane protein and a member of the toll-like receptor family, and increases ACE2 expression. This further assists in SARS-CoV-2 infection.
Importantly, myocarditis and hyperinflammation, found in severely infected COVID-19 patients, may occur due to over-activation of TLR4 by the spike glycoprotein, which results in excessive release of pro-inflammatory cytokines downstream of TLR4.
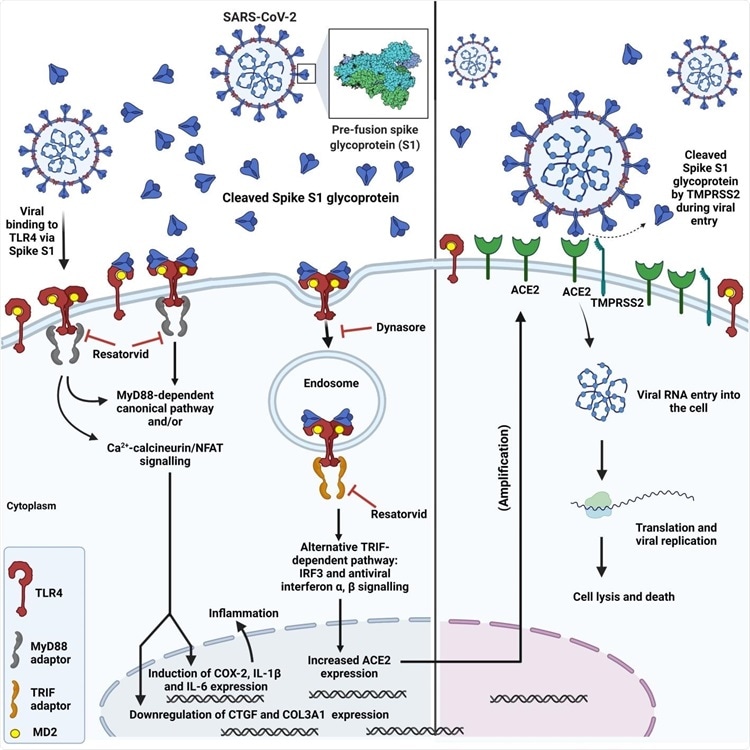 Diagram showing SARS-CoV-2 spike interaction with TLR4, subsequent TLR4 activation via canonical and endosomal pathways and induction of inflammation, upregulation of ACE2 expression and increased viral entry.
Diagram showing SARS-CoV-2 spike interaction with TLR4, subsequent TLR4 activation via canonical and endosomal pathways and induction of inflammation, upregulation of ACE2 expression and increased viral entry.
The study team re-evaluated their previously proposed hypothesis, which stated that the TLR4 activated by the spike glycoprotein S1 domain increases ACE2 expression and, thereby, aids in COVID-19 infection.
The study team conducted in vitro studies using rat and human cells to test the original hypothesis.
The study findings revealed that the spike S1 domain of SARS-CoV-2 is a TLR4 agonist in rat and human cells, which is responsible for stimulating a pro-inflammatory M1 macrophage phenotype in human THP-1 monocyte-derived macrophages.
Typically the SARS-CoV-2 transmembrane spike glycoprotein contains two subunits, namely, S1 and S2.
However for this study, the externally protruding S1 subunit was only considered because based on in silico molecular simulations; it contains the putative binding site to TLR4 and the Receptor Binding Domain (RBD), which binds to ACE2.
Past studies have revealed that the S1 domain initially binds to ACE2 via the RBD. In contrast, the S2 domain mediates the fusion of the virus and host cell membranes, thereby leading to the entry of the virus into the host cell.
However the study findings show that the S1 also activates TLR4 in neighboring cells during the initial infection. It has also highlighted the significance of the process of spike S1 domain being externalized from the cell.
The study findings highlight that the spike S1 binds and activates TLR4 to increase ACE2 expression and triggers the release of pro-inflammatory cytokines. This was demonstrated using adult rat cardiac tissue-resident macrophage-derived cells (cTMFs). cTMFs express TLR4 and co-express macrophage and myofibroblast markers.
The study team also used human cells expressing TLR4 (hTLR4-HA HEK 293 cells) and human monocyte-derived macrophages that express TLR4 to confirm their hypothesis.
The study team reported that the SARS-CoV-2 spike S1 subunit not only binds and activates TLR4, but also upregulates ACE2 expression in rat cTMFs.
This study finding was derived using various techniques such as RT-qPCR, immunoblotting, confocal immunofluorescence microscopy, and proximity ligation assay.
Furthermore the study findings also showed that the spike S1 caused downstream effects, analogous to bacterial lipopolysaccharide (LPS) on the expression of some inflammatory and fibrotic markers, e.g., cyclo-oxygenase-2 (COX-2), connective tissue growth factor (CTGF), and the collagen 3a1 isoform (COL3A1).
The study team further reported that the effect of the spike S1 domain was inhibited by the selective TLR4 signaling inhibitor CLI-095 (Resatorvid® or TAK-242) which confirms that S1 is a TLR4 agonist and a viral PAMP for TLR4.
It must be noted that the binding of spike S1 to TLR4 in rat cTMFs and human TLR4 expressing cells was determined using proximity ligation assays. Further, inhibition of the expression of ACE2 by the dynamin inhibitor Dynasore® indicated that the endosomal/b-interferon pathway facilitates this expression.
Also the study team pointed out that confocal immunofluorescence microscopy helped determine 1:1 stoichiometric spike S1 co-localization with TLR4 in rat and human cells.
The study team also reported that spike S1/IFN-γ treatment of THP-1-derived macrophages triggers the pro-inflammatory M1 polarization, which was indicated by an increase in IL-1β and IL-6 mRNA.
Importantly the study findings present evidence for the further development of TLR4 antagonist drugs to improve the treatment of COVID-19 by reducing viral entry and inflammation.
For more on
COVID-19 Immunology, keep on logging to Thailand Medical News.

