University of California Study Uncovers The Dual Nature of Proliferating Plasmablasts in Dengue and COVID-19 Infections
Thailand Medical News Aug 12, 2023 2 years, 4 months, 2 weeks, 1 day, 15 hours, 49 minutes ago
COVID-19 Research: In the realm of infectious diseases, the immune system's response holds the key to understanding the intricacies of pathogenesis and potential avenues for therapeutic intervention. Two notable viral infections, dengue fever and COVID-19, have recently captured global attention due to their widespread impact and diverse clinical manifestations. B cells, essential components of the immune response, play a pivotal role in these diseases. A deeper exploration of B cell dynamics, particularly focusing on proliferating plasmablasts, reveals an intriguing Janus-faced aspect that has far-reaching implications for disease severity and immunological memory.
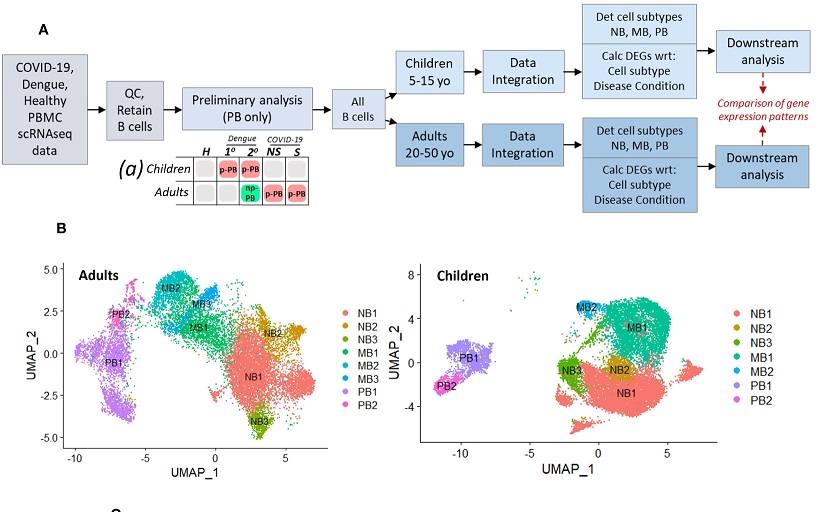 Schematic describing experimental design, with preliminary analysis and subsequent parallel analysis of pediatric and adult age groups, for disease conditions: primary dengue (1°), secondary dengue (2°), non-severe COVID-19 (NS), severe COVID-19 (S), age-matched healthy controls (H). Chart (a) describes results of preliminary analysis: red boxes represent presence of proliferative plasmablast (p-PB) population, green box represents non-proliferative (np-PB) population, grey boxes represent insufficient number of plasmablasts. (B). UMAP embeddings for all B cells from adults (left) and children (right), showing cell subsets within naive B cells (NB), memory B cells (MB), and plasmablasts (PB).
B Cells in Dengue and COVID-19
Schematic describing experimental design, with preliminary analysis and subsequent parallel analysis of pediatric and adult age groups, for disease conditions: primary dengue (1°), secondary dengue (2°), non-severe COVID-19 (NS), severe COVID-19 (S), age-matched healthy controls (H). Chart (a) describes results of preliminary analysis: red boxes represent presence of proliferative plasmablast (p-PB) population, green box represents non-proliferative (np-PB) population, grey boxes represent insufficient number of plasmablasts. (B). UMAP embeddings for all B cells from adults (left) and children (right), showing cell subsets within naive B cells (NB), memory B cells (MB), and plasmablasts (PB).
B Cells in Dengue and COVID-19
Dengue fever, caused by the dengue virus with its four serotypes, has long been a concern in tropical and subtropical regions. On the other hand, the emergence of COVID-19, resulting from the novel coronavirus SARS-CoV-2, has caused a global pandemic. While the two viruses differ in their modes of transmission and target cells, both infections manifest dysregulation of B cell subsets, including naive B cells, memory B cells, and plasmablasts (PBs).
Notably, both dengue and COVID-19 exhibit a surge in circulating plasmablasts during acute infection, and this increase in PBs has been correlated with disease severity.
Unveiling Proliferating Plasmablasts
Recent investigations, employing the power of single-cell RNA sequencing (scRNAseq), have unearthed a heterogeneous landscape within the peripheral plasmablast population in COVID-19 patients. Different subsets of plasmablasts have been identified, characterized by distinct attributes such as proliferation, robust interferon response, and heightened unfolded protein response, which supports antibody production. A parallel scenario unfolds in dengue fever, where the presence of proliferative pre-plasmablasts in the circulation has been noted. This observation opens the gateway to understanding the mechanisms underpinning disease severity.
Exploring B Cell Dynamics
The quest to decipher the intricate interactions of B cells in the context of dengue and COVID-19 led the
COVID-19 Research team to delve into the mechanistic underpinnings that drive the expansion of proliferative plasmablast populations. Comparative analysis of scRNAseq datasets from pediatric and adult patients infected with
either dengue or COVID-19 illuminated intriguing patterns. The distinct presence of proliferative plasmablasts was noted in pediatric dengue cases, particularly secondary infections, and in severe cases of COVID-19 in adults. Conversely, this population was notably absent in adults with dengue and children with COVID-19. This distinctive signature propelled further investigation into naive and memory B cells.
Unraveling the Molecular Tapestry
A detailed scrutiny of gene expression patterns within naive and memory B cells shed light on the molecular tapestry underlying the emergence of proliferating plasmablasts. Notable differences emerged in cell sensing, activation, and downstream signal transduction via the B cell receptor and Rho GTPases. The inflammatory landscape also bore unique imprints, with the pro-inflammatory S100 gene family and interferon-stimulated genes taking center stage in cases with proliferative plasmablasts. Notably, transcription factors acting as mediators of crosstalk between inflammation, cell fate decisions, and proliferation exhibited distinct expression patterns in the presence and absence of proliferative plasmablasts.
Implications and Future Avenues
The dynamic interplay between B cell activation, inflammation, and transcriptional regulation offers a compelling narrative to explain the emergence of proliferating plasmablasts in the circulation. While the current study employs dengue and COVID-19 as model infections, the implications extend far beyond these specific diseases. The intricate connections between pro-inflammatory cues and lineage determination in naive and memory B cells provide valuable insights into the mechanisms of immune memory, vaccine development, and post-viral autoimmune syndromes.
Challenges and Future Directions
Despite the strides made in unraveling the complexities of B cell dynamics, challenges remain. The intricate balance between peripheral blood B cells and those within secondary lymphoid organs poses a significant hurdle. As lymphocyte activation and maturation primarily occur within these organs, a comprehensive understanding necessitates a broader exploration of multiple cell types involved in B cell responses. Moreover, the study's reliance on peripheral blood samples underscores the need for a more nuanced approach that captures the early stages of illness to decipher cell specification dynamics.
Conclusion
In the realm of infectious diseases, the dual nature of proliferating plasmablasts in dengue and COVID-19 infections unveils a captivating tale of immune response and disease severity. This intricate interplay between naive and memory B cells, inflammation, and transcriptional regulation provides a window into the mechanisms driving the emergence of proliferative plasmablasts. As we unravel these mysteries, we pave the way for more effective therapeutic strategies, enhanced vaccine development, and a deeper understanding of the complex landscape of immunological memory. The journey into the world of B cells continues to captivate researchers, offering a promise of better control over viral infections and their aftermath.
The study findings were published in the peer reviewed journal: Frontiers In Immunology.
https://www.frontiersin.org/articles/10.3389/fimmu.2023.1068424/full
For the latest
COVID-19 Research, keep on logging to Thailand Medical News.
