University Of California Study Validates That SARS-CoV-2 Causes Neuroinflammation
Source: COVID-19 Neuroinflammation Nov 04, 2022 2 years, 5 months, 2 weeks, 2 days, 5 hours, 51 minutes ago
COVID-19-Neuroinflammation: A new study by researchers from the University of California- Davis, USA involving rhesus macaques (Macaca mulatta) of Indian origin has demonstrated that SARS-CoV-2 infections induces neuroinflammation.
 Graphical Abstract
Graphical Abstract
The study findings in a non-human monkey models have strong implications of the effects of SARS-CoV-2 on the brains of humans.
The SARS-CoV-2 coronavirus, the etiologic agent of coronavirus disease 2019 (COVID-19), can induce a plethora of neurological complications in some patients.
It is still under debate however as to whether SARS-CoV-2 directly infects the brain or whether CNS sequelae result from systemic inflammatory responses triggered in the periphery.
The
COVID-19-Neuroinflammation study team by utilizing high-resolution microscopy, investigated whether SARS-CoV-2 reaches the brain and how viral neurotropism can be modulated by aging in a non-human primate model of COVID-19.
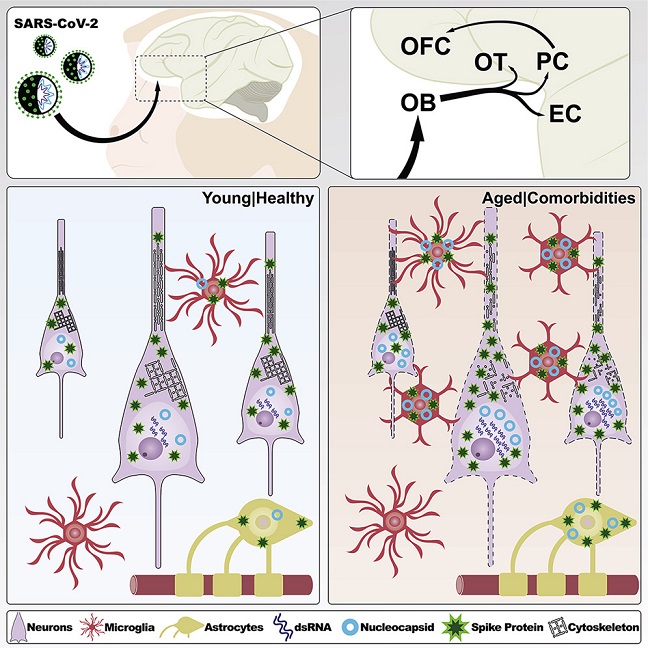
The study findings showed that seven days after infection, SARS-CoV-2 was detected in the olfactory cortex and interconnected regions and was accompanied by robust neuroinflammation and neuronal damage exacerbated in aged, diabetic animals.
The study findings provide an initial framework for identifying the molecular and cellular mechanisms underlying SARS-CoV-2 neurological complications, which will be essential to reducing both the short- and long-term burden of COVID-19.
The study findings were published in the peer reviewed journal: Cell Reports.
https://www.cell.com/cell-reports/fulltext/S2211-1247(22)01434-6
This is the first study to employed a non-human monkey model for COVID-19 to examine whether SARS-CoV-2 penetrates the brain and the impact of aging on viral neurotropism.
The study team used 14 colony-bred male and female rhesus macaques (Macaca mulatta) of Indian origin. The two main age groups for the animals were young adults aged between four and 12 years and older subjects aged 18 to 24. Three subgroups of young adults and older subjects were identified: infected non-type-2 diabetics, non-infected type-2 diabetics, and non-infected non-diabetics.
For the study, clinical observation includes keeping track of a research specimen's response, discharge, breathing rate and pattern, coughing and sneezing, appetite, and stool quality was assessed. Furthermore, rectal temperature, sp02, respiration, heart rate, and skin turgor were among the additional clinical evaluations performed. The investigation was further expanded to incorporate more brain areas and several viral markers. The study team assessed neuroinflammation in the COVID-19 rhesus model developed in the study.
The piriform cortex was extensively studied since it consistently showed the mos
t significant levels of SARS-CoV-2 spike (Spk) and double-stranded ribonucleic acid (dsRNA). The team proposed that SARS-CoV2 neuroinflammation could cause cellular and synaptic damage.
Study findings involving cell-type analysis showed that in the young as well as old animals, the SARS-CoV-2 nucleocapsid (N) immunosignal colocalized with astrocytes (GFAP+), microglia (Iba1+), and neurons (NeuN+).
It was also found that neurons had the highest level of intracellular N protein irrespective of age. At the same time, elderly animals revealed higher quantities than young animals across all three cell types, according to volume quantification of intracellular N protein by cell type.
The study findings imply that SARS-CoV-2 exhibits some neurotropism and that the infection burden rises with age.
Spike protein, as well as dsRNA, an intermediary component in the SARS-CoV-2 replication cycle that acts as a surrogate for productive infection, were also detected with SARS-CoV-2 N.
Importantly, immunolabeling for these markers was found in multiple areas of the primary olfactory cortex, including the olfactory tubercle, the piriform cortex, and the olfactory pole of the entorhinal cortex, irrespective of age. In aged animals, SARS-CoV-2 viral markers were also observed in the orbitofrontal cortex, which is part of the secondary olfactory cortex. In contrast, only minimal labeling was observed outside the primary olfactory cortex in young animals.
The study findings showed that SARS-CoV-2 was neurotropic and could be identified in the macaque brain's olfactory regions within seven days post-infection (dpi).
Though both glial and neuronal cells have viral proteins, productive infection is predominantly neuronal. Aged T2D mice showed more significant cellular changes in response to the virus as well as increased viral loads in all areas analyzed.
Interestingly, when young, infected animals were compared to their corresponding age-matched, non-infected controls, there was a rise in the number of astrocytes. Notably, GFAP populations significantly increased in both young and old infected mice, suggesting either astrocyte growth or translocation to the piriform cortex.
It was also noted that microglial proliferation/translocation showed a considerable increase among aged and infected animals. These study findings point to a significant inflammatory response to SARS-CoV-2 infection that involves the mobilization of both astrocytic and microglial cascades. Interestingly, only the older mice had inflammatory changes linked to diabetes, as evidenced by assessing the piriform cortex of diabetic young and elder control animals.
Also, it was found that human leukocyte antigen-D-related+ (HLA-DR) microglia displayed significant correlations with degraded myelin essential protein (dgMBP) instead of regular MBP expression, which could result in white-matter injury.
The HLA-DR+ microglia were in contact with neurons that expressed major histocompatibility complex (MHC) class I, which was a viral antigen that was significantly elevated in neurons present in diabetic older animals but worsened after SARS-CoV-2 infection.
The study team also noted a higher frequency associated with microglia’s abnormal morphology among infected animals. Notably, these microglia were found in the vicinity of neurons having morphological alterations, thus indicating a significant impact that suggested a neurodegenerative process.
When compared to young infected animals, there was a considerable drop in the density of PSD95+ puncta in older infected mice. The total microglia cellular volume was also higher in aged, infected animals as compared to young and infected subjects as well as aged, non-infected controls.
Significantly, the total amount of internalized pan-Neurofilament (pan-NF) components increased in elderly, infected microglia.
The research findings revealed the prevalence of productive neuronal infection, along with the limited distribution of SARS-CoV-2 viral proteins into the olfactory circuit, indicated rapid transneuronal viral spread across corticocortical pathways, resulting in its dissemination in the CNS through the olfactory connectome.
The study findings can also help explain why older humans with diabetes might be more vulnerable to SARS-CoV-2 infections.
The key findings of the study were:
-In macaques, SARS-CoV-2 is found in olfactory brain areas at 7 days post infection
-Neurons are initially the primary target of SARS-CoV-2 productive infection
-Neurocovid is accompanied by robust neuroinflammation and vascular disruption
-SARS-CoV-2 brain pathology is worsened by aging and diabetes in infected monkeys
For more on
COVID-19-Neuroinflammation, keep on logging to Thailand
Medical News.
