University of Warwick Develops New And More Accurate Diagnostic Technology Using Sugars To Detect SARS-COV-2 Coronavirus
Source: COVID-19 Diagnostics Oct 19, 2021 4 years, 2 months, 2 days, 12 hours, 16 minutes ago
COVID-19 Diagnostics: Scientist from the department of chemistry at the University of Warwick-UK along with experts from Iceni Diagnostics Ltd-UK and University Hospitals Coventry and Warwickshire (UHCW) NHS Trust-UK have developed and demonstrated a new diagnostic technology using sugars instead of antibodies to detect the SARS-CoV-2 coronavirus.
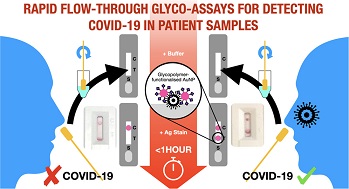
To date since the start of the COVID-19 pandemic, various RT-PCR platforms and also antigen test kits as been used as the main diagnostic tools.
LFDS or Lateral-flow diagnostics are rapid and of lower cost than molecular (genetic) tests.
However most current LFDs are using antibodies as their recognition units.
The
COVID-19 Diagnostics study team developed a prototype flow-through device (related, but distinct to LFDs), utilizing N-acetyl neuraminic acid-functionalized, polymer-coated, gold nanoparticles as the detection/capture unit for SARS-COV-2, by targeting the sialic acid-binding site of the spike protein. The prototype device can give rapid results, with higher viral loads being faster than lower viral loads. The prototype’s effectiveness is demonstrated using spike protein, lentiviral models, and a panel of heat-inactivated primary patient nasal swabs.
The study findings showed that the device was able to retain detection capability toward recombinant spike proteins from several variants (mutants) of concern.
These findings provide the proof of principle that glyco-lateral-flow devices could be developed to be used in the tracking monitoring of infectious agents, to complement, or as alternatives to antibody-based systems.
The study findings were published in the peer reviewed journal: ACS Sensors
https://pubs.acs.org/doi/10.1021/acssensors.1c01470
The lateral flow diagnostics (LFDs) have been widely used during the COVID-19 pandemic to provide rapid identification of individuals with an active infection. These LFDs work by using antibodies, which 'stick' to the SARS-COV-2 virus.
The study team lead by Professor Dr Matthew Gibson from the University of Warwick along with experts from Iceni Diagnostics developed an alternative system of detection using glycans ('sugars'), where synthetic polymer chains are used to attach the glycans to the surface of nanoparticles.
Typically, viruses use glycans as a 'handle' to attach to our cells, and the study team mimicked this process to enable detection of SARS-COV-2.
Along with medical teams from the UHCW NHS Trust, the study team demonstrated that prototype devices could identify COVID-19 positive swabs across a range of viral loads accurately.
.jpeg) Nanoparticle synthesis and flow-through devices. (A) Neu5NAc-terminated polymer coating; (B) TEM micrograph of polymer-coated AuNPs; (C) C
1s portion of the XPS spectrum of polymer-coated AuNPs; and (D) flow-through device layout and assay procedure (top to bottom).
Nanoparticle synthesis and flow-through devices. (A) Neu5NAc-terminated polymer coating; (B) TEM micrograph of polymer-coated AuNPs; (C) C
1s portion of the XPS spectrum of polymer-coated AuNPs; and (D) flow-through device layout and assay procedure (top to bottom).
The study team also demonstrated that the technology functioned well with the spike proteins from variants of concern, which is a key benefit of using glycan-binding technology.
The study findings clearly demonstrate that glycan-recognition technology can be used to identify pathogens, which the academic/industry team are actively developing further as part of a collaborative project.
The study findings show the potential of using glycans as alternative detection reagents, compared to the traditional antibody-based techniques.
Dr Matthew Gibson, Professor at Warwick Medical School and the Department of Chemistry, University of Warwick added, “The use of our polymeric linkers, which allows us to present the glycan on the nanoparticles (which make the red line), shows the benefit of true cross-disciplinary, cross-sector collaboration. This work shows that our approach can work with primary clinical samples and we are actively developing this into a real-world device with our partners."
Dr Dimitris Grammatopoulos, Professor at Warwick Medical School and Consultant in Clinical Biochemistry at UHCW NHS Trust, further added, "This is a testament to the cutting-edge scientific research taking place at the University of Warwick and UHCW NHS Trust. Initial results of this prototype showed it can perform favorably in comparison to established COVID-19 tests with respect to cost, time, accuracy and reliability. We are delighted to collaborate on this research."
Iceni Diagnostics' Chief Scientific Officer Professor Dr Rob Field, commented, "Our ambition was to exemplify how an academic and industry collaboration can translate hard-core scientific discoveries into practical solutions, and this study has proven we can do this by combining our deep experience in glycoscience. The successful testing of the prototype device and glycan-based platform will now enable us to progress our viral and other pathogen pipelines, and we are delighted to continue working with the University of Warwick team on this program."
The study team concluded, “The evidence provided here shows that glycan flow technology (lateral-flow and flow-through glyco-assays) could be translated to clinical settings to be used alongside more traditional antibody-based approaches.”
Commercial production of the new test kits are already under production and various tests and clinical setting studies are currently being conducted in order for the new diagnostics to be approved by regulatory bodies in the United States and also in Europe.
For more on the latest
COVID-19 diagnostics, keep on logging to Thailand Medical News.

.jpeg) Nanoparticle synthesis and flow-through devices. (A) Neu5NAc-terminated polymer coating; (B) TEM micrograph of polymer-coated AuNPs; (C) C
1s portion of the XPS spectrum of polymer-coated AuNPs; and (D) flow-through device layout and assay procedure (top to bottom).
Nanoparticle synthesis and flow-through devices. (A) Neu5NAc-terminated polymer coating; (B) TEM micrograph of polymer-coated AuNPs; (C) C
1s portion of the XPS spectrum of polymer-coated AuNPs; and (D) flow-through device layout and assay procedure (top to bottom).