Updates On Coronavirus Vaccine Progress And The New Broad Spectrum Vaccine Approach
Source: Thailand Medical News Feb 07, 2020 5 years, 11 months, 3 weeks, 5 days, 20 hours, 31 minutes ago
The sudden emergence of the respiratory
coronavirus in China has once again got global biotech and medical researchers rushing to develop a
vaccine against the fast spreading and deadly disease. However no one can predict when it would materialize as there are so many factors affecting its development.
Immediately after researchers and health officials from China disseminated the genetic profile of the new
coronavirus to counterparts globally, biotech specialists at the U.S. National Institutes of Health started the process of developing a critical component for a
vaccine, planning to start initial testing and clinical trials as early as April.
Australian and French medical researchers also joined the race to develop and effective
vaccine against the
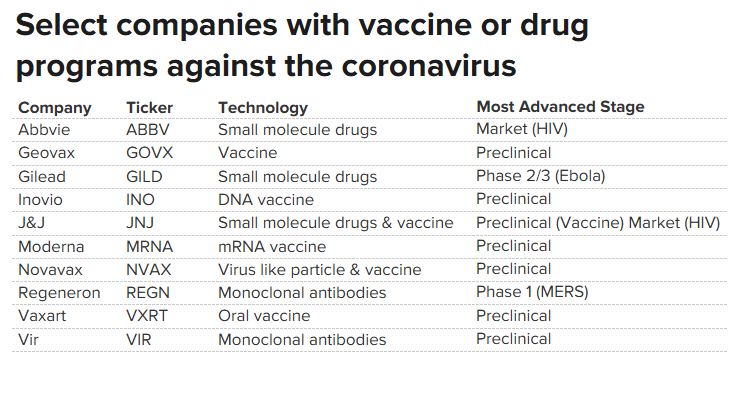
coronavirus with many biotech and vaccine companies trying out different innoculation platforms and strategies. Among the pharma and biotech companies are Moderna Therapeutics. Inovio Pharmaceuticals, Johnson & Johnson, Novavax, Gilead and many more.
Interestingly, biotech researchers from Texas researchers had frozen an experimental
vaccine developed during the time of the SARS outbreak but did not get a chance to use as the epidemic died down naturally by itself. They are now trying to get U.S. and Chinese health officials to perform clinical trials on it. Dr. Peter Hotez of Baylor College of Medicine and Texas Children's Hospital who was part of the research team that had developed the
vaccines explained that as the SARS and the 2019-NCoV
coronavirus are from the same family and have about 82 to 87 percent likeness, it has a very high possibility of working.
Even though the progress of the
coronavirus vaccine is moving at a very fast phase as a result of past data from the SARS and MERS outbreaks and research, it might still take a year before a proper effective vaccine materializes according to almost all the experts from the various teams. And by then it is not sure if its use is still warranted as the epidemic could have died down or the virus could have mutated drastically.
Currently more than 31,800 people are infected with the
coronavirus in China alone with more than 650 deaths while in the rest of the world there are about 240 cases of confirmed infections and two deaths so far.
At the moment, the strategy of health authorities are to isolate the infected to prevent further spread of the coronavirus which manifest symptoms such fever, flu-like conditions, cough and in severe cases pneumonia.
As ther
e are no specific drugs to date, Chinese doctors had been experimenting with various antiviral drugs including those used in HIV. However it is noticed that the
coronavirus is also beginning to exhibit resistance to some of these antivirals, which is alarming medical researchers.
Ready Made Broad Spectrum Vaccines
Dr Peter Hotez, who created the earlier SARS
vaccine with Texas Children' Hospital colleague Dr Maria Elena Bottazzi told
Thailand Medical News, "Our
vaccine is already manufactured and could proceed very quickly also there's fixed no road map or set of directions for what one needs to do to make a vaccine in the midst of a massive deadly viral outbreak."
Specialists from NIH are advocating that rather than going after outbreaks, it is better to pursue
broad spectrum vaccines that could work for entire virus families, ready to be used any time a new viral disease emerges.
Dr. Barney Graham, deputy director of the National Institute of Allergy and Infectious Diseases'
Vaccine Research Center commented, "Currently the world has the technology now to develop
broad spectrum vaccines. It is a more better option from an engineering and biological standpoint. Not doing so makes us at risk again for a new and next pandemic.”
Jump Start Procedures
In the past, developing
vaccines required first growing lots of virus in a lab which could be time consuming.
The biotech researchers at NIH have developed a novel faster method: By utilizing a piece of the virus' genetic code, called messenger RNA or mRNA, that instructs cells to make a particular protein.
Dr. Tal Zaks, CMO of Moderna Inc., which is developing mRNA
vaccines for other diseases and is currently working with NIH on the new
coronavirus vaccine, told
Thailand Medical News, "We think of RNA as the software of life,
by simply injecting the right genetic piece, one can teach the body to make its own medicine. As cells produce just that protein, the immune system learns to recognize it, primed to attack if the entire virus ever comes along.”
He further explained, “The main target is a protein aptly named ‘spike’ that lets the virus bind to cells. It studs the surface of
coronaviruses, the new one as well as its cousins SARS, which erupted in China in 2002 and spread to 26 countries, and MERS, or Middle East respiratory syndrome, which emerged in 2012.”
Dr Graham's research team focused in on the RNA responsible for the new virus' production of spike and then because prior research showed the protein can alter its shape, engineered a more stable version of it.
Pharma giant, Moderna is making samples of the synthetic mRNA
vaccine for NIH to use initially in animal studies and within three months initiate its first-stage safety tests in people. If further testing proves it really works, the hope is biotech engineers could simply swap in a new spike code if another
coronavirus comes along.
This is significant and critical as after a few such outbreaks in less than 20 years, it is not going to be the last for the
coronaviruses according to Professor Dr Mark Denison, a virologist at Vanderbilt University Medical Center.
He added. “It is important to develop
vaccine strategies that go after these unique things common to every
coronavirus."
Other Vaccine Developments
Meanwhile Inovio Pharmaceuticals strategy to
vaccine development is to approach with synthetic DNA. Inovio recently reported promising results from initial -stage testing of a MERS vaccine.
Inovio is now collaborating with a Chinese company, Beijing Advaccine, to conduct human clinical trials in China by the third quarter of 2020.
French researchers at the Pasteur Institute are focusing a strategy from the
vaccine against measles. The French biotech scientists had some early progress concocting genetic material from other viruses into that
vaccine, and now plan to activate the immune system against the new
coronavirus in the same manner.
Virologist Dr Frederic Tangy, head of Pasteur's vaccine innovation department said, "The approach we are doing now involves developing a
vaccine against measles but having it re-engineered so that it has antigens from the new
coronavirus".
As far as the previous SARS
vaccine that was developed by Dr. Peter Hotez of Baylor College of Medicine, it is actually developed on a similar concept of a spike protein being genetically engineered and grown in a lab, what can be termed as a rather traditional approach compared to the newer and less proven Moderna and Inovio strategies. It did show protection in tested animals but have yet to progress to human clinical trials as the Texas researchers had ran out of funds. However, the team ensures that their sample stocks are still well preserved for usage.
Going After Epidemic Outbreaks
Previous viral epidemic outbreaks are full of such missed chances to develop a
broad spectrum vaccine. Currently there is no commercial
vaccine for MERS even though sporadic outbreaks occur. The birth defect-causing Zika outbreak was ending by the time human trials could have started for a
vaccine.
However Ebola
vaccines provide some optimism. Some
vaccine types started early trials during the massive Ebola epidemic in West Africa between 2015-2016, although the outbreak waned before biotech researchers had clinical evidence they worked. However health officials and biotech companies kept up the research, and in 2018 when another outbreak happened in Congo, these
vaccines were ready for use just in time.
WHO or the World Health Organization officials will be convening next week to identify promising treatment protocols, drug and
vaccine candidates for the new
coronavirus and fast-track their development.
However the key consensus among the medical and biotech community is that an approach towards
broad spectrum vaccines for the
coronavirus is the best way.
For more drug, vaccine or information about the
coronavirus epidemic or the
Thailand Coronavirus scenario, keep on checking
at:
https://www.thailandmedical.news/articles/coronavirus 
