US COVID-19 News: US FDA Stops Antibody Blood Test Kits Pending Proof Of Efficacy While Many Countries Are Finding Kits From China To Be Faulty or Fake
Source: US COVID-19 News May 05, 2020 5 years, 7 months, 3 weeks, 18 hours, 27 minutes ago
US COVID-19 News: The US FDA yesterday (4
th April 2020) retracted an earlier approval that allowed scores of COVID-19 antibody blood tests kits to hit the market without first providing proof that they worked.
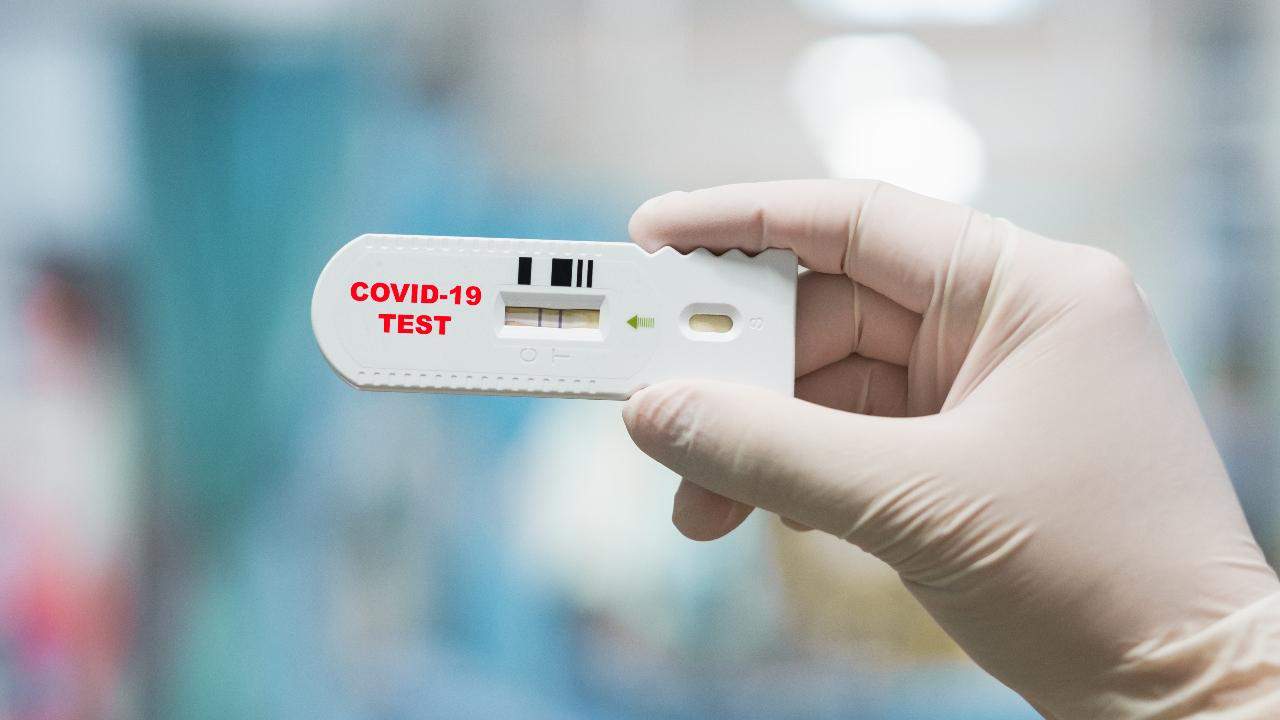
The US regulators said it took the action because some sellers have made false claims about the tests and their accuracy. All manufacturers or distributors will now have to show their tests kits work or risk having them banned from the market.
The US FDA in March initially allowed companies to begin selling tests as it was under pressure to increase testing options. The US FDA stipulated that as long as these companies notified the agency of their plans and provided disclaimers, including that they were not FDA approved., it was ok. The policy was intended to allow "flexibility" needed to quickly ramp up production.
Dr Anand Shah, an FDA deputy commissioner, said, "However, flexibility never meant we would allow fraud. We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans."
Antibody blood tests are different from the nasal swab tests currently used to diagnose active COVID-19 infections. Instead, the tests look for blood proteins called antibodies, which the body produces days or weeks after fighting an infection. Most use a finger-prick of blood on a test strip.
The newly revised policy follows weeks of criticism from doctors, lab specialists and members of Congress who said the FDA's lack of oversight created a Wild West of unregulated tests.
The US FDA acknowledged yesterday that there have been problems with deceptive, false marketing among the 160 tests that have been launched in the U.S. Some companies have claimed their tests can be used at home, although FDA has not allowed that use. Others make unsubstantiated claims about their accuracy.
Many hospitals and local governments have reported buying tests that turned out to be inaccurate or frauds.
To date, the US FDA has granted authorization to only 12 antibody tests, meaning their methods, materials and accuracy passed muster with agency regulators. Companies with test kits currently on the market without FDA authorization will now be required to submit formal applications to regulators within 10 business days. Companies that launch at a later date will have 10 days to turn over their applications after validating their tests.
Initially health officials in America and around the world have suggested the tests could be helpful in identifying individuals who have previously had the virus with or without getting sick and developed some immunity to it. But medical researchers have not yet been able to answer key questions that are essential to their practical use: what level of antibodies does it take to be immune and how long does that protection last?
Dr Kamran Kadkhoda, a lab director at the Cleveland Clinic said, We are spending a lot of time and resources on something that is not really a panacea for reopening."
Currently the tests are mainly a research tool for scientists trying to determine how widely the SARS-CoV-2 coronavirus has spread amon
g the American population. Those studies are underway but have produced widely different preliminary results, in part, due to variations between tests. Even high-performing tests can produce skewed results when used in a large population where few people have had the virus.
The NIH and other federal agencies are also reviewing tests and conducting research into whether they can successfully predict immunity.
Dr Stephen Hahn, US FDA Commissioner told media that his agency's "careful balancing of risks and benefits shifted to the approach we've outlined today," based on new data from FDA and NIH reviews. Hahn said more than 200 companies are in the process of submitting testing data to the FDA.
Medical experts who criticized the government's previous policy welcomed the new evidence requirement.
Dr Robin Patel of the Mayo Clinic, "We want to make sure that testing in the U.S. is of high quality and that those using the tests understand how the results should or should not be used."
Meanwhile health authorities and medical experts around the world are warning countries and medical institutions to avoid buying antibody test kits from China as almost all the products coming from China were found to be either faulty and in most cases simply fake.
Even more dangerous is the fact that some of these Chinese manufacturers are now dumping these faulty or fake medical stocks onto various online e commerce platforms targeting end consumers as a means to sell off their stocks. The public are warned about buying any medical devices, equipment or products from online e-commerce platforms as most of them originating from China are substandard and certain e-commerce sites will not even tell you that it is originating from China.
For the latest
US COVID-19 news, keep on logging to Thailand Medical News.
A Call For Help! Please help support to sustain this website and all our efforts to propel further research and also various international community projects by making a donation. Donations are accepted via paypal. https://www.thailandmedical.news/p/sponsorship
