US FDA Flags Heart Balloon Pump That Has Caused 2 More Recent Death, To date 60 Device Failures Reported
Sourec: Thailand Medical News Nov 24, 2019 6 years, 2 months, 1 week, 6 days, 19 hours, 31 minutes ago
-Two more deaths bring the total to about 7 this year alone, and more than 60
medical device reports have been linked to battery issues with Getinge’s Maquet/Datascope
intra-aortic balloon pump (IABP) over the past year, US
FDA said last week in a letter to healthcare providers and hospitals.
-
Getinge subsidiary Maquet recalled almost 23,000 of the pumps in May due to the reports of battery failures. US
FDA issued a notification on the recall in July. At that time, the agency said five deaths had been tied to the devices since March 2016.
-US
FDA’s latest warning gives an update on a problem first communicated in November 2018, when the agency warned it had received 75 reports in two years of the device shutting down while operating on battery power.
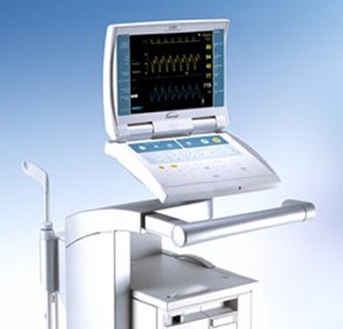
Numerous technical problems have been reported with the Maquet/Datascope devices, including failure to hold a battery charge, sudden shutdowns, and shortened battery run times that could cause the pump to stop working. In its update, FDA said it wants to make sure healthcare providers are aware that device failures continue to be reported in patients treated with Getinge’s IABPs.
US FDA said it remains concerned about the shutdowns associated with the cardiac assist devices but recognizes that the systems may be the best option for circulatory support for some patients. The pumps treat patients undergoing surgery or those with acute coronary syndrome or complications from heart failure.
Maquet/Datascope is contacting customers to schedule training on updated battery instructions, use and maintenance. A reference guide based on the operating instruction manual for each IABP also is now provided with each device.
In updates as well as in its earlier communications, FDA said the patient deaths could not be attributed with certainty to the device shutting down. But because the IABP devices support critically ill patients both in healthcare facilities and during transport, an interruption in treatment could cause serious harm or death.
Maquet/Datascope is in the process of developing a battery maintenance software upgrade, FDA said. The agency said it continues to work with the company to determine why the devices may shut down while running on battery power.
The IABP devices affected by the problem are the Cardiosave Hybrid and Rescue, CS300 and CS100/CS100i. A software upgrade was released for the CS300 and CS100/CS100i in 2017.
Thailand Medical News has no updates about the product recall from
hospitals in Asia that are using the devices. According to undiclosed sources, there are about 13,700 of these devices in 128 hospitals in Asia.
Reference:
https://www.fda.gov/medical-devices/letters-health-care-providers/update-device-failure-associated-getinges-maquetdata
scope-intra-aortic-balloon-pumps-letter-health 