West Virginia University Researchers Discover Way to Open Blood-Brain Barrier Using Ultrasound
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 11, 2025 11 months, 1 week, 6 days, 14 hours, 13 minutes ago
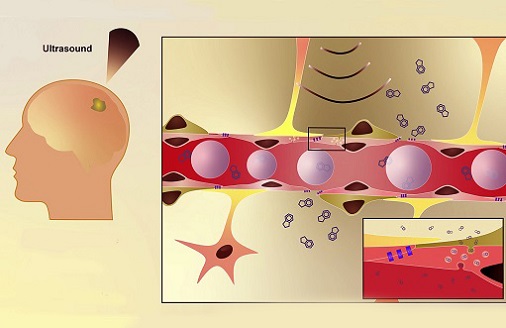
Medical News: The blood-brain barrier (BBB) serves as a critical gatekeeper, protecting the brain from potentially harmful substances while permitting the transport of essential nutrients. However, this protective shield poses significant challenges for treating central nervous system (CNS) diseases, as it often restricts the delivery of therapeutic drugs. Researchers at West Virginia University’s School of Pharmacy and Rockefeller Neuroscience Institute have explored a non-invasive technique known as low-intensity focused ultrasound (LiFUS) to temporarily open the BBB. The study aimed to investigate the immediate and secondary effects of this method, providing insights into how BBB permeability, transporter function, and inflammatory responses are influenced.
 West Virginia University Researchers Discover Way to Open Blood-Brain Barrier Using Ultrasound
How the Research Was Conducted
West Virginia University Researchers Discover Way to Open Blood-Brain Barrier Using Ultrasound
How the Research Was Conducted
The study involved a meticulous set of experiments to examine the effects of LiFUS on the BBB. Researchers used in situ brain perfusion to assess changes in vascular permeability and glucose uptake while evaluating the function of a crucial protein transporter called P-glycoprotein (P-gp). Additionally, they performed cytokine analysis to measure inflammatory responses in the brain tissue. Both wild-type and immunodeficient mice were studied to identify variations in immune responses.
The research team - including experts from the Department of Neuroscience and the Department of Neuroradiology - used advanced MRI-guided ultrasound technology. The LiFUS treatment was paired with microbubbles to enhance its efficacy. This
Medical News report focuses on the key findings and implications of the study.
Key Findings of the Study
One of the most significant findings was the biphasic nature of BBB opening observed after a single exposure to LiFUS. Researchers noted two distinct phases of increased BBB permeability. The first phase occurred immediately after sonication, characterized by a temporary disruption in BBB integrity. The second phase, occurring six hours post-treatment, revealed additional permeability increases accompanied by marked changes in glucose uptake and vascular volume.
Enhanced Glucose Uptake and Energy Demands
The study highlighted a sharp rise in glucose uptake six hours after LiFUS exposure, suggesting heightened energy requirements for repair and recovery processes in brain cells. The increased activity of GLUT-1 transporters - proteins responsible for glucose transport—may indicate that neurons, astrocytes, and endothelial cells were engaging in repair mechanisms. This finding underscores the metabolic changes induced by BBB disruption and its potential therapeutic implications.
Reduced P Glycoprotein Activity
Another intriguing observation was the reduction in P-glycoprotein (P-gp) function six hours post-LiFUS. P-gp serves as a molecular pump, clearing drugs and toxins from the brain. While its normal activity helps maintain brai
n health, it can also hinder the effectiveness of CNS-targeted therapies. The transient reduction in P-gp function during the secondary phase of BBB opening could present a valuable therapeutic window for enhancing drug delivery to the brain.
Neuroinflammation and Immune Responses
The researchers also investigated inflammatory responses associated with LiFUS-induced BBB disruption. Significant elevations in pro-inflammatory cytokines, such as TNF-α, IL-6, and CXCL1, were detected in the sonicated brain regions six hours post-treatment. Interestingly, some of these inflammatory markers were also elevated in the contralateral (non-sonicated) brain regions and in the bloodstream, though to a lesser degree. These findings suggest that even adjacent brain regions may experience secondary effects from BBB disruption.
The study further revealed differences in inflammatory responses between wild-type and immunodeficient mice. Wild-type mice exhibited higher levels of inflammatory cytokines, including TNF-α and IFN-γ, compared to their immunodeficient counterparts. This difference highlights the influence of immune status on the brain’s response to LiFUS, providing valuable insights for tailoring therapeutic strategies.
Implications for CNS Drug Delivery
The ability to temporarily and selectively open the BBB using LiFUS has significant implications for treating CNS diseases such as Alzheimer’s, Parkinson’s, and brain tumors. By exploiting the secondary phase of BBB opening, when permeability is heightened, and P-gp activity is reduced, clinicians may achieve better drug delivery outcomes. However, the inflammatory responses observed in the study underscore the need for caution, as excessive neuroinflammation could exacerbate underlying conditions.
Study Limitations and Future Directions
While this research represents a significant step forward, the authors acknowledge several limitations. For instance, changes in the expression of certain BBB transporters, such as JAMs and OATPs, were not fully explored.
Additionally, the precise mechanisms driving the biphasic nature of BBB opening remain unclear. Future studies should investigate these aspects and explore how genomic and proteomic changes contribute to the observed phenomena. Further research is also needed to understand the long-term effects of repeated LiFUS exposure.
Conclusions
The findings of this study provide a deeper understanding of the biphasic nature of BBB opening induced by LiFUS. By characterizing the timing and extent of secondary physiological changes, the research paves the way for optimizing therapeutic strategies for CNS diseases. The insights into reduced P-gp activity and increased glucose uptake during the secondary phase offer potential for enhancing drug delivery efficacy. However, the observed inflammatory responses highlight the importance of monitoring and mitigating potential adverse effects.
With its ability to transiently disrupt the BBB in a controlled and reversible manner, LiFUS holds great promise as a tool for CNS drug delivery. This work emphasizes the need for a balanced approach that maximizes therapeutic benefits while minimizing risks.
The study findings were published in the peer-reviewed journal: Pharmaceutics.
https://www.mdpi.com/1999-4923/17/1/75
For the latest on the Blood-Brain Barrier, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/researchers-discover-way-to-allow-drugs-to-penetrate-through-the-blood-brain-barrier
https://www.thailandmedical.news/news/australian-study-finds-that-those-with-post-covid-cognitive-issues-are-likely-to-suffer-from-blood-brain-barrier-disruption-and-glutamatergic-excitoto
https://www.thailandmedical.news/news/covid-19-causes-disruptions-in-blood-brain-barrier-via-mitochondria-impairment-and-endothelial-dysfunction
https://www.thailandmedical.news/news/scientists-from-singapore-discover-plasma-biomarkers-that-identify-blood-brain-barrier-dysregulation-in-alzheimer-s-disease
