Nikhil Prasad Fact checked by:Thailand Medical News Team May 24, 2024 11 months, 2 weeks, 2 days, 9 hours, 42 minutes ago
Cancer News: Prostate cancer remains one of the most prevalent forms of cancer among men in the United States. According to the American Cancer Society, nearly 300,000 new cases are diagnosed each year, affecting approximately one in eight men over their lifetimes. Traditional treatments, such as androgen deprivation therapy (ADT), aim to lower testosterone levels to shrink tumors. However, these treatments often come with significant side effects, including sexual dysfunction and weight gain. Additionally, many patients eventually develop castrate-resistant prostate cancer (CRPC), a more lethal form of the disease that continues to grow even when testosterone levels are reduced. Amidst this challenge, a team of researchers at the Medical College of Wisconsin has identified a novel treatment protocol that may revolutionize prostate cancer therapy.
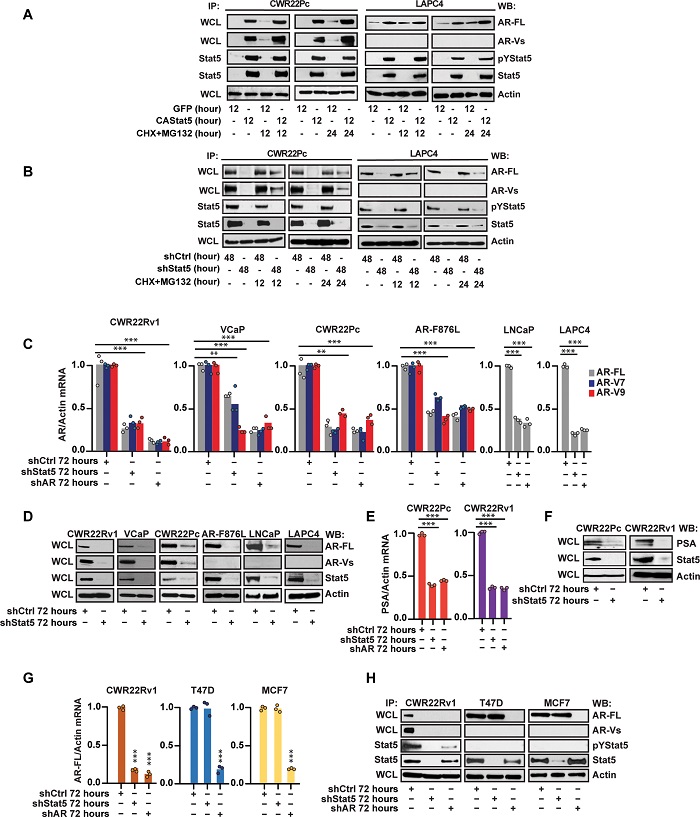 Active Stat5 increases protein levels of full-length androgen receptor (AR-FL) and AR variants (AR-Vs) through the induction of AR mRNA levels in PC. A) CA Stat5a/b or green fluorescent protein (GFP) was lentivirally expressed in CWR22Pc and LAPC for 12 hours followed by CHX (30 μM) and MG132 (10 μM) for 12 or 24 hours. AR protein levels were determined by Western blotting (WB) of whole-cell lysates (WCLs) with actin as a loading control. Active Stat5 levels were determined by immunoprecipitation (IP) of Stat5 followed by WB for phosphorylated Stat5 (pStat5) and total Stat5 (A, B, D, F, and H). (B) Stat5 was suppressed by lentiviral shStat5 expression in CWR22Pc and LAPC4 cells for 48 hours followed by CHX (30 μM) and MG132 (10 μM) for 12 or 24 hours. (C) Lentiviral shStat5 expression with control (shCtrl) for 72 hours in CWR22Rv1, VCaP, CWR22Pc, and ENZ-resistant CWR22Pc-expressing AR-F876L, LNCaP, and LAPC4. AR-FL, AR-V7, and AR-V9 mRNA levels were determined by qRT-PCR with lentiviral shRNA depletion of AR (shAR) for 72 hours as control. (D) The same cell lines were transduced by lenti-shStat5 for 72 hours followed by the IP of AR-FL, AR-V, Stat5, and actin by IP and WB. (E) Lentiviral transduction of Stat5 shRNA for 72 hours in CWR22Pc and CWR22Rv1 cells in comparison to lenti-shCtrl or lenti-shAR followed by the determination of PSA mRNA and protein (F) levels by qRT-PCR and WB. AR was suppressed by the lentiviral expression of shAR as a control for qRT-PCR. (G) Lentiviral expression of shStat5 versus shCtrl or shAR for 72 hours in T47D, MCF7, and CWR22Rv1 cells followed by the determination of AR mRNA and protein (H) levels by qRT-PCR and WB using lenti-shAR as control.
A New Therapeutic Approach
Active Stat5 increases protein levels of full-length androgen receptor (AR-FL) and AR variants (AR-Vs) through the induction of AR mRNA levels in PC. A) CA Stat5a/b or green fluorescent protein (GFP) was lentivirally expressed in CWR22Pc and LAPC for 12 hours followed by CHX (30 μM) and MG132 (10 μM) for 12 or 24 hours. AR protein levels were determined by Western blotting (WB) of whole-cell lysates (WCLs) with actin as a loading control. Active Stat5 levels were determined by immunoprecipitation (IP) of Stat5 followed by WB for phosphorylated Stat5 (pStat5) and total Stat5 (A, B, D, F, and H). (B) Stat5 was suppressed by lentiviral shStat5 expression in CWR22Pc and LAPC4 cells for 48 hours followed by CHX (30 μM) and MG132 (10 μM) for 12 or 24 hours. (C) Lentiviral shStat5 expression with control (shCtrl) for 72 hours in CWR22Rv1, VCaP, CWR22Pc, and ENZ-resistant CWR22Pc-expressing AR-F876L, LNCaP, and LAPC4. AR-FL, AR-V7, and AR-V9 mRNA levels were determined by qRT-PCR with lentiviral shRNA depletion of AR (shAR) for 72 hours as control. (D) The same cell lines were transduced by lenti-shStat5 for 72 hours followed by the IP of AR-FL, AR-V, Stat5, and actin by IP and WB. (E) Lentiviral transduction of Stat5 shRNA for 72 hours in CWR22Pc and CWR22Rv1 cells in comparison to lenti-shCtrl or lenti-shAR followed by the determination of PSA mRNA and protein (F) levels by qRT-PCR and WB. AR was suppressed by the lentiviral expression of shAR as a control for qRT-PCR. (G) Lentiviral expression of shStat5 versus shCtrl or shAR for 72 hours in T47D, MCF7, and CWR22Rv1 cells followed by the determination of AR mRNA and protein (H) levels by qRT-PCR and WB using lenti-shAR as control.
A New Therapeutic Approach
Dr Marja Nevalainen, a translational medicine physician-scientist, led the groundbreaking research. She explained to
Cancer News journalists at TMN, "We want to develop a new therapeutic approach for androgen deprivation in a way that is more patient-friendly. The innovative treatment focuses on manipulating cell signaling pathways to curb tumor growth more effectively and with fewer side effects.”
Androgen deprivation th
erapy traditionally targets the androgen receptor, a crucial protein involved in testosterone signaling and prostate tumor growth. However, over time, the androgen receptor can mutate, rendering ADT ineffective. Dr Nevalainen’s approach involves blocking the activity of Stat5, a protein that boosts androgen receptor levels and promotes prostate cancer growth.
The Role of Stat5 in Prostate Cancer
Stat5, comprising two highly homologous isoforms, Stat5a and Stat5b, acts both as a cytoplasmic signaling protein and a nuclear transcription factor. When activated by phosphorylation, Stat5 forms dimers that translocate to the nucleus and activate specific genes that support prostate cancer cell viability and growth. Previous studies have shown that blocking Stat5 signaling can induce the death of prostate cancer cells and suppress tumor growth.
The study team developed a drug that inhibits Stat5, and their research demonstrated that inhibiting Stat5 significantly slowed tumor growth and decreased androgen receptor levels in cell cultures and human tumors grafted into mice. This approach, by suppressing the androgen receptor through Stat5 inhibition, potentially carries a lower risk of developing castrate-resistant prostate cancer, as the androgen receptor is less likely to mutate or adapt genetically.
Translating Research to Clinical Application
The next step for the study team is to translate these promising results from preclinical models to human clinical trials. A new drug influencing Stat5 activity is currently being tested in Phase II clinical trials. This development could mark a significant milestone in prostate cancer treatment, offering a more effective and patient-friendly alternative to existing therapies.
A Closer Look at Androgen Deprivation Therapy
The primary protein driving prostate cancer growth and progression is the androgen receptor (AR), a transcription factor induced by androgenic steroids like testosterone. Targeting AR signaling through ADT is a cornerstone of treatment for advanced prostate cancer or cases where the tumor recurs after surgery. ADT can be implemented in various ways: suppressing circulating androgen levels, inhibiting androgen synthesis, or directly inhibiting the AR with high-affinity antagonists.
However, despite initial effectiveness, ADT often leads to the emergence of castrate-resistant prostate cancer (CRPC), characterized by tumors reactivating AR through multiple mechanisms. In a significant proportion of CRPC cases, amplification of the AR gene increases AR expression, including splice variants that display constitutive transcriptional activity. Other mechanisms include structural rearrangements of the AR gene and somatic AR point mutations, which broaden the repertoire of ligands activating the AR.
Stat5: A Druggable Pathway to Target AR Signaling
Dr Nevalainen's research discovered a previously unknown role of activated Stat5 signaling as a robust inducer of AR gene transcription in prostate cancer. This finding opens new therapeutic avenues, suggesting that pharmacological inhibition of Stat5 could suppress AR signaling and prostate cancer growth more effectively.
The Stat5 inhibitor developed by the study team team, IST5-002, disrupts Stat5 activity at nanomolar concentrations. Their studies showed that IST5-002 suppresses AR levels and tumor growth in various prostate cancer models, including castrate-resistant forms. By down-regulating Stat5 activity, the inhibitor reduces the levels of wild-type, mutated, and truncated AR proteins.
Genetic and Pharmacological Insights
Genetic knockdown of Stat5 consistently suppressed both AR and AR splice variant mRNA levels in human prostate cancer cell lines, irrespective of somatic AR mutations or gene amplification status. The extent of AR suppression by Stat5 depletion was comparable to direct genetic knockdown of AR itself. Furthermore, Stat5 knockdown decreased AR mRNA levels in cell lines engineered to express clinical AR gene rearrangements.
Activated Stat5 not only induced AR mRNA and protein levels but also promoted AR signaling in prostate cancer cells. This was demonstrated through various experimental setups, including cytokine activation of the Jak2-Stat5 pathway and overexpression of activated Stat5. Interestingly, while Stat5 depletion affected AR stability, activation of Stat5 did not increase AR protein stability, suggesting tissue-specific mechanisms.
Broader Implications and Future Directions
The findings from Dr Nevalainen’s research highlight the potential of Stat5 inhibitors like IST5-002 in providing a new therapeutic strategy for prostate cancer. These inhibitors could offer more prolonged clinical responses by reducing the levels of diverse AR protein species, thereby mitigating the adaptive changes that drive prostate cancer progression.
Future studies will need to explore the tissue-specific effects of Stat5 induction and its implications across various AR-positive cell types, including normal and malignant tissues. Additionally, investigating the epigenetic modifications and Stat5-regulated AR repressors in prostate cancer cells will provide deeper insights into the mechanisms underlying Stat5's role in AR gene transcription.
Overall, the identification of Stat5 as an inducer of AR gene transcription in prostate cancer presents a promising new avenue for therapy. By targeting this pathway, researchers hope to develop treatments that are not only more effective but also more tolerable for patients, ultimately improving the prognosis for those diagnosed with this prevalent and challenging disease.
The study findings were published in the peer reviewed journal: Science Advances.
https://www.science.org/doi/10.1126/sciadv.adi2742
For the latest
Cancer News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/university-of-michigan-develops-new-urine-test-that-detects-high-grade-prostate-cancer-as-an-alternative-to-biopsies
https://www.thailandmedical.news/news/herbs-and-phytochemicals-alpha-santalol-from-sandalwood-can-prevent-development-of-prostate-cancer
https://www.thailandmedical.news/news/medical-news-singapore-doctors-warn-that-acute-covid-19-infections-can-cause-elevated-psa-levels,-complicating-prostate-cancer-diagnosis
https://www.thailandmedical.news/news/medical-news-uk-study-finds-that-carnosine-could-be-used-to-treat-prostate-cancer
https://www.thailandmedical.news/news/herbs-and-phytochemicals-daidzein-and-genistein-from-soyabeans-and-equol,-a-gut-flora-metabolite-from-daidzein-can-help-in-prostate-cancer
