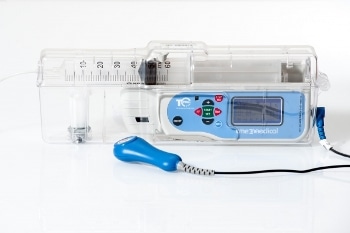
The TPCA™ Ambulatory Patient Controlled (PCA) syringe pump with lockbox has a library of protocols offering MediGuard, a Dose Error Reduction System (DERS) which can be set to the patients needs or pain management speciality in hospital and homecare environments.
Features
Main Features
- Lightweight and compact, suitable for both bedside and ambulatory use
- Rechargeable Li-Polymer battery or mains power allows greater flexibility
- Integral protective syringe cover
- Can be programmed to bolus only, basal and bolus, basal only
- Code protected clinician bolus function
- Can be configured with up to six protocols to accommodate local policy and procedure
- Syringe recognition of commonly used syringe brands (2 to 50 ml)
- Can be pole mounted or portable
- Suitable for adults or for paediatric use
- Automatic bolus rate adjustment optimises bolus delivery and prevents false occlusion alarms
- Two protocols that can be modified via the pump by authorised personnel
- Lockbox
- Shift total screen
Safety Features
- Fixed or variable code protected protocols that are configured and locked into the pump memory and can be accessed by authorised personnel or technicians
- Current infusion history can be viewed during infusion
- Pump event log accessible via INFO MENU or downloaded to PC through BodyComm software
- Comprehensive range of alerts / alarms with descriptive display of state and resolution
- Lockable keypad to prevent inadvertent key activation
- MediGuard™ weight-based dosing software will automatically set a toxicity ceiling based on your patient’s weight, to prevent excess drug delivery
- Residual patient bolus screen display
Accessories and Consumables
Accessories
- D7140: Clear Front Single Use Pouch (fits pump in Lockbox)
- D7139: Solid Front Single Use Pouch (fits pump in Lockbox)
- 100-174SL: T60/TPCA/T34 50 ml Lockbox + 2 Keys
- 141-100XM: Remote Bolus Button - Coded
- 100-099SLA: Dongle, IR220, RS232 Fixed Baud Rate
- 150-311CPC: Docking Station RS232 Lead
- USB2: USB Serial Adapter
- 151-132X: UK Wall Charger -8.2 V
- 400-651SL: T60 Pole Mounting Device
- BODYCOMM1: Bodycomm TPCA/T60 - No Leads
- BODYCOMM2: Bodycomm TPCA/T60 - With RS232 Lead
- BODYCOMM3: Bodycomm TPCA/T60 - With USB Adapter
- BCS100: Bodycomm 100 Software
Consumables
- Please contact a member of our Customer Support Team on 01253 206700 for more information about administration sets for the TPCA.
Technical Specification
| Device type | Syringe pump with motor driven linear actuator, pulsed motion (60 pulses per mm) |
| Basal rate | 0.1 to 650 ml/h |
| Bolus rate range | 0 – 1000 ml/h |
| Actuator stroke | c.107 mm available |
| Syringe sizes | 2 ml to 60 ml (pump configured to most commonly used syringes including BD Plastipak, Monoject, Braun, Terumo and Codan syringes but can also be programmed to other brands) |
| Pump accuracy | +/- 2% |
| Occlusion pressure | 100 to 1500 mmHg. Max actuator force 50 N (5 Kgf) |
| Battery | Rechargeable Li-Polymer 7.4 volt, 1800 mAh |
| Battery operation | 100 full deliveries depending on infusion and set up parameters |
| Battery charging | Automatic when connected to an AC power source |
| Indicators | Graphic LCD display (128 x 64 pixels with backlight) , dual color operation LED to denote infusion and alarm status and keypad |
| Alarms | When a problem is detected, the TCPA displays the following alarm messages, sounds an audible alarm & the red LED lights - occlusion, syringe empty, near end, end battery, locking cover open, low battery, pump paused too long, syringe displaced, syringe error |
| TPCA dimensions | 38 x 55 x 190 mm (L x W x H) |
| Classification | Type CF Equipment, degree of protection against electrical shock Class II equipment, IPX3 protection against ingress of water |
| Housing | Polycarbonate ABS (fire retardant) |
| Weight | 400 g with battery |
| Electrical safety | IEC 60601-2-24 (Infusion pumps and controllers), IEC 60601-1-4 (Programmable Electrical Medical System), UL 60601- 1 and CAN/CSA C22.2 No 601.1 |
| Standards | Manufactured in accordance to ISO 9001 and ISO 13485. CE marked (in accordance with the Medical Devices Directive 93/42/EEC) |
| EMC | IEC 60601-1-2 (EMC) |
| Environmental specifications | Non operating conditions (transportation and storage) |
| Temperature -25 ° to +50 °C (-13 °F to +122°F) | |
| Humidity 5% to 100% R.H, non-condensing | |
| Air pressure 48 kPa to 110 kPa | |
| Operating Conditions | The system may not meet all performance specifications if operated outside of the following conditions |
| Temperature +15 °C to +45 °C (+59 °F to +113 °F) | |
| Humidity 20% to 85% R.H at +40 °C, non-condensing | |
| Air pressure 70 kPa to 110 kPa |
For more detail: http://www.cmemedical.co.uk/product/tpca-patient-controlled-analgesia-pca-syringe-pump-lockbox/